
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.3, Problem 1.13P
Characterize each of the following structures as aromatic, nonaromatic, or antiaromatic:
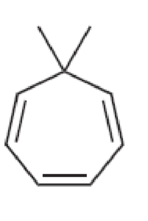
Answer: _____
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please correct answer and don't use hand rating
5
Aromatic molecules must be
Aromatic Rules:
суclic
planar
fully conjugated
contain 4n + 2n electrons
Answer Bank
contain an odd number of pairs of a electrons
асyclic
contain 4n a electrons
contain an even number of pairs of a electrons
non-planar
Incorrect
Chapter 1 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 1.2 - Prob. 1.2PCh. 1.2 - Prob. 1.3PCh. 1.2 - Prob. 1.4PCh. 1.2 - Prob. 1.5PCh. 1.2 - Prob. 1.6PCh. 1.3 - Characterize each of the following structures as...Ch. 1.3 - Characterize each of the following structures as...Ch. 1.3 - Characterize each of the following structures as...Ch. 1.3 - Characterize each of the following structures as...Ch. 1.3 - Characterize each of the following structures as...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. A schedule of experiments for a laboratory indicates that the 1-bromobutane preparation is paired with the p...
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Explain why 92% of 2,4-pemtanedione exists as the enol tautomer in hexane but only 15% of this compound exists ...
Organic Chemistry
In qualitative analysis, Ca2+ and Ba2+ are separated from Na+, K+, and Mg2+ by adding aqueous (NH4)2CO3 to a so...
General Chemistry: Atoms First
APPLY 1.2 Express the following quantities in scientific notation
using fundamental SI units of mass and lengt...
Chemistry (7th Edition)
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
18.23 Do the reactions involved in ozone depletion involve changes in oxidation state of the O atoms? Explain.
Chemistry: The Central Science (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Classify each of the following compounds as aromatic or non-aromatic. SH D C Structure A V[Choose ] Aromatic Non-aromatic Structure B Choose] Structure C [Choose ] Structure D [ Choose ) Structure E [ Choose] Structure F Choose1 B.arrow_forwardAromaticity and Properties Identify the properties that describe aromatic compounds. Aromatic Answer Bank planar all single bonds flexible rigid twisted alternating double and single bonds electrons fully delocalized in a ring all bond lengths between double and single bond lengths all double bonds reactive unpaired electrons very unstable very stablearrow_forwardDetermine if the following compounds are not aromatic, aromatic or anti-aromatic. :O: 00 IZ:arrow_forward
- Define if AROMATIC, ANTI AROMATIC, OR NON AROMATICarrow_forwardComplete the structure of the species given below based on the formula below thecompounds. Identify the number of π electrons in each compound and indicate if thecompound is aromatic, non-aromatic or anti-aromatic.arrow_forwardplease help with this question. thank you. Classify the following compounds (if flat) as aromatic, antiaromatic, or nonaromatic.arrow_forward
- Which of the following compounds is/are aromatic? OFF GUN TAY NEW Select one: O OFF and TAY OFF and NEW OFF and GUN GUN and TAYarrow_forwardIdentify the properties that describe aromatic compounds.arrow_forward4) Classify the compound below as aromatic, anti- aromatic, or non-aromatic. Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY