
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12C.9, Problem 22P
How many signals are present in the
a. 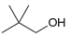
b. 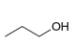
c. 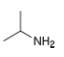
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Show work
Is this aromatic, antiaromatic, or nonaromatic?
On what basis are Na and Nb ranked against each other?
Chapter 12C Solutions
Organic Chemistry (6th Edition)
Ch. 12C.1 - Problem 14.1 The NMR spectrum of recorded on a ...Ch. 12C.1 - Prob. 2PCh. 12C.2 - How many 1H NMR signals does each...Ch. 12C.2 - How many 1H NMR signals does each compound give?...Ch. 12C.2 - Label the protons in each highlighted CH2 group as...Ch. 12C.2 - How many 1H NMR signals would you expect for each...Ch. 12C.3 - Prob. 9PCh. 12C.3 - For each compound, first label each different type...Ch. 12C.3 - Label each statement as True or False. a. When a...Ch. 12C.4 - Prob. 12P
Ch. 12C.5 - Problem 14.12 Which compound give a NMR spectrum...Ch. 12C.5 - Prob. 14PCh. 12C.6 - Prob. 15PCh. 12C.6 - For each compound give the number of 1H NMR...Ch. 12C.6 - Prob. 17PCh. 12C.7 - Prob. 18PCh. 12C.7 - Problem 14.18 Describe the NMR spectrum of each...Ch. 12C.8 - Problem 14.19 Draw a splitting diagram for in ,...Ch. 12C.8 - Problem 14.20 Identify A and B, isomers of...Ch. 12C.9 - Problem 14.21 How many signals are present in the ...Ch. 12C.9 - Problem 14.22 What protons in alcohol A give rise...Ch. 12C.9 - How many peaks are observed in the NMR signal for...Ch. 12C.10 -
Problem 14.24 Propose a structure for a compound...Ch. 12C - 14.34 (a) How many NMR signals does each of the...Ch. 12C - 14.35 (a) How many NMR signals does each compound...Ch. 12C - Prob. 38PCh. 12C - 14.37 How many NMR signals does each natural...Ch. 12C - Prob. 40PCh. 12C - 14.39 What effect does increasing the operating...Ch. 12C - Prob. 43PCh. 12C - Prob. 44PCh. 12C - Prob. 45PCh. 12C - Prob. 47PCh. 12C - Prob. 48PCh. 12C - 14.48 How many NMR signals does each compound...Ch. 12C - 14.49 Rank the highlighted carbon atoms in each...Ch. 12C - 14.50 Identify the carbon atoms that give rise to...Ch. 12C - 14.62 Reaction of with , followed by treatment...Ch. 12C - Reaction of aldehyde D with amino alcohol E in the...Ch. 12C - 14.64 Propose a structure consistent with each set...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Step 1: add a curved arrow. Select Draw Templates More / " C H Br 0 Br : :o: Erase H H H H Q2Q Step 2: Draw the intermediates and a curved arrow. Select Draw Templates More MacBook Air / " C H Br 0 9 Q Erase 2Qarrow_forwardO Macmillan Learning Question 23 of 26 > Stacked Step 7: Check your work. Does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? Draw the expected product of the forward reaction. - - CN DMF MacBook Air Clearly show stereochemistry. Questionarrow_forwardNH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forward
- Predict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forwardIntercalation compounds have their sheetsa) negatively charged.b) positively charged.arrow_forward
- Indicate whether the following two statements are correct or not:- Polythiazine, formed by N and S, does not conduct electricity- Carbon can have a specific surface area of 3000 m2/garrow_forwardIndicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forwardcould someone draw curly arrow mechanism for this question pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY