
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12C.5, Problem 13P
Which compound give a
a.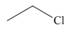 b.
b.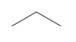 c.
c.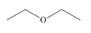 d.
d.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
can you please help me solve hw
Give a brief comparison of chemical potential and electrochemical potential.
can you please help me solve hw
Chapter 12C Solutions
Organic Chemistry (6th Edition)
Ch. 12C.1 - Problem 14.1 The NMR spectrum of recorded on a ...Ch. 12C.1 - Prob. 2PCh. 12C.2 - How many 1H NMR signals does each...Ch. 12C.2 - How many 1H NMR signals does each compound give?...Ch. 12C.2 - Label the protons in each highlighted CH2 group as...Ch. 12C.2 - How many 1H NMR signals would you expect for each...Ch. 12C.3 - Prob. 9PCh. 12C.3 - For each compound, first label each different type...Ch. 12C.3 - Label each statement as True or False. a. When a...Ch. 12C.4 - Prob. 12P
Ch. 12C.5 - Problem 14.12 Which compound give a NMR spectrum...Ch. 12C.5 - Prob. 14PCh. 12C.6 - Prob. 15PCh. 12C.6 - For each compound give the number of 1H NMR...Ch. 12C.6 - Prob. 17PCh. 12C.7 - Prob. 18PCh. 12C.7 - Problem 14.18 Describe the NMR spectrum of each...Ch. 12C.8 - Problem 14.19 Draw a splitting diagram for in ,...Ch. 12C.8 - Problem 14.20 Identify A and B, isomers of...Ch. 12C.9 - Problem 14.21 How many signals are present in the ...Ch. 12C.9 - Problem 14.22 What protons in alcohol A give rise...Ch. 12C.9 - How many peaks are observed in the NMR signal for...Ch. 12C.10 -
Problem 14.24 Propose a structure for a compound...Ch. 12C - 14.34 (a) How many NMR signals does each of the...Ch. 12C - 14.35 (a) How many NMR signals does each compound...Ch. 12C - Prob. 38PCh. 12C - 14.37 How many NMR signals does each natural...Ch. 12C - Prob. 40PCh. 12C - 14.39 What effect does increasing the operating...Ch. 12C - Prob. 43PCh. 12C - Prob. 44PCh. 12C - Prob. 45PCh. 12C - Prob. 47PCh. 12C - Prob. 48PCh. 12C - 14.48 How many NMR signals does each compound...Ch. 12C - 14.49 Rank the highlighted carbon atoms in each...Ch. 12C - 14.50 Identify the carbon atoms that give rise to...Ch. 12C - 14.62 Reaction of with , followed by treatment...Ch. 12C - Reaction of aldehyde D with amino alcohol E in the...Ch. 12C - 14.64 Propose a structure consistent with each set...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Reason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forwardIn electrochemistry, briefly describe the Galvani potential, the Volta potential, and the surface potential. Differentiate between them.arrow_forward
- What substances can neutralize, complex or adsorb and absorb both HF and CF carbonyl fluoride and hydrogen fluoride and intermediate formation of thermal decomposition of fluorinated inorganic compounds either due to hydrolysis and hygroscopic reactions. What is the known chemistry of these reactions and mechanisms.arrow_forwardBriefly differentiate between chemical potential and electrochemical potential.arrow_forwardAccording to open access forums ionic antimony Sb (111) can be reduced to elemental Sb (0) in solution and in macromolecules like condensation polymers polyethylene terephthalate (PET) causing greying of the polymer matrix. It has been connected to thermal degradation of the polymer during processing to the formation of thermally unstable EG ethyleen glycol that forms at various temperatures formic acid, formaldehyde, acetaldehyde and much more depending on temperature. I need to know what organics are more powerful reducing agents and at what concentration (relative) to each organic will initiate this reduction. Furthermore, is the pH dependant ? Are other trace elements in the plastic also a cause of concern e.g. aluminum from aluminum chloride (lewis acid). Therefore, the ultimate solution should include a means to inhibit reduction of ionic antimony and will the same solution comply with cobalt impurities from ionic cobalt? Some PET have combinations of catalyst and their residues…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY