
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
4th Edition
ISBN: 9780134639673
Author: Elizabeth A. Stephan, David R. Bowman, William J. Park, Benjamin L. Sill, Matthew W. Ohland
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12.5, Problem 9CC
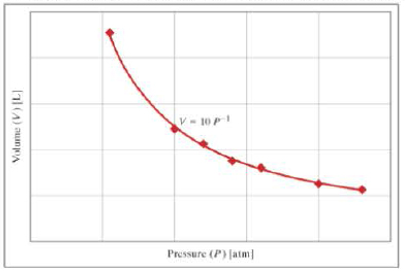 The graph shows the ideal gas law relationship (PV=nRT) between pressure (P) and volume (V). If the tank is at a temperature of 300 kelvins and is filled with nitrogen (formula, N2; molecular weight, 28 grams per mole), what is the mass of gas in the tank in units of grams?
The graph shows the ideal gas law relationship (PV=nRT) between pressure (P) and volume (V). If the tank is at a temperature of 300 kelvins and is filled with nitrogen (formula, N2; molecular weight, 28 grams per mole), what is the mass of gas in the tank in units of grams?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Compute the mass fraction of eutectoid cementite
in an iron-carbon alloy that contains 1.00 wt% C.
Compute the mass fraction of eutectoid cementite
in an iron-carbon alloy that contains 1.00 wt% C.
!
Required information
Mechanical engineering, don't use
chatgpt.
Thanks
A 60-kip-in. torque T is applied to each of the cylinders shown.
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
3 in.
4 in.
(a)
(b)
Determine the inner diameter of the 4-in. diameter hollow cylinder shown, for which the maximum stress is the same as in part a.
The inner diameter is
in.
Chapter 12 Solutions
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Ch. 12.3 - Fluid A as a dynamic viscosity of 0.5 centipoise...Ch. 12.3 - Fluid A has a dynamic viscosity of 0.5 centipoise...Ch. 12.3 - Fluid A has a dynamic viscosity of 0.5 centipoise...Ch. 12.4 - You have three springs, with stiffness 1,2 and 3...Ch. 12.4 - You have three resistors with resistance 2,2, and...Ch. 12.4 - You have four 60-nanofarad [nF] capacitors. Using...Ch. 12.4 - You have three 120 millihenry [mH] inductors. Can...Ch. 12.5 - The graph shows the ideal gas law relationship...Ch. 12.5 - The preceding graph shows the ideal gas Jaw...Ch. 12.6 - The decay of a radioactive isotope was tracked...
Ch. 12 - The graph shows the ideal gas law relationship (PV...Ch. 12 - An inductor is an electrical device that can store...Ch. 12 - Solid objects, such as your desk or a rod of...Ch. 12 - Mercury has a dynamic viscosity of 1.55...Ch. 12 - SAE 99W10, a brand new type of motor oil has a...Ch. 12 - You have two springs each of stiffness 2 newton...Ch. 12 - Prob. 7ICACh. 12 - Four springs were tested, with the results shown...Ch. 12 - Four circuits were tested, with the results shown...Ch. 12 - Assume you have an unlimited number of inductors...Ch. 12 - a. The equivalent capacitance of the circuit shown...Ch. 12 - A standard guitar, whether acoustic or electric,...Ch. 12 - The vibrating frequency of a guitar string depends...Ch. 12 - Solid objects, such as your desk or a rod of...Ch. 12 - Eutrophication is a process whereby lakes,...Ch. 12 - The following graph shows the relationship between...Ch. 12 - The total quantity (mass) of a radioactive...Ch. 12 - Match the data series from the options shown on...Ch. 12 - 1. For a simple capacitor with two f lat plates,...Ch. 12 - 2. When we wish to generate hydroelectric power,...Ch. 12 - 3. When rain falls over an area for a sufficiently...Ch. 12 - You are experimenting with several liquid metal...Ch. 12 - 5. The resistance of a wire (R [ohm)) is a...Ch. 12 - 6. Use the figure shown to answer the following...Ch. 12 - 7. You are given four springs, one each of...Ch. 12 - You have three springs. You conduct several tests...Ch. 12 - 9. You are given four resistors, each of 7.5, 10,...Ch. 12 - 10. You have three resistors. You conduct several...Ch. 12 - 11. Use the diagrams shown to answer the following...Ch. 12 - 12. When a buoyant cylinder of height H, such as a...Ch. 12 - 13. It is difficult to bring the Internet to some...Ch. 12 - 14. The data shown in the following graph was...Ch. 12 - 15 A standard guitar, whether acoustic or...Ch. 12 - 16. Your supervisor has assigned you the task of...Ch. 12 - 17. One of the NAE Grand Challenges for...Ch. 12 - 18. When volunteers build a Habitat for Humanity...Ch. 12 - 1. As part of an electronic music synthesizer, you...Ch. 12 - Prob. 20RQ
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
The solid steel shaft AC has a diameter of 25 mm and is supported by smooth bearings at D and E. It is coupled ...
Mechanics of Materials (10th Edition)
A nozzle at A discharges water with an initial velocity of 36 ft/s at an angle with the horizontal. Determine ...
Vector Mechanics For Engineers
This optional Google account security feature sends you a message with a code that you must enter, in addition ...
SURVEY OF OPERATING SYSTEMS
How is the hydrodynamic entry length defined for flow in a pipe? Is the entry length longer in laminar or turbu...
Fluid Mechanics: Fundamentals and Applications
Look at the following description of a problem domain:
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
How are relationships between tables expressed in a relational database?
Modern Database Management
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Mechanical engineering, Don't use chatgpt. Strict warning.arrow_forward10:38 PM P 4136 54 A man Homework was due west for and 4km. He then changes directies walks on a bearing south-wes IS How far Point? of 1970 until he of his Starting Port Is he then from his stating What do you think about ... ||| Մ כarrow_forwardA simply supported T-shaped beam of 6m in length has to be designed to carry an inclined central point load W. Find the max- imum value of this load such that the maximum tensile and com- pression stresses on the beam do not exceed 30 and 60 respectively. N mm² N mm², 90 mm 80 mm Y W 60 mm 30° 10 mm 10 mm Xarrow_forward
- Problem 9.5 9.5 A 1080-kg car is parked on a sloped street. The figure shows its wheels and the position of its center of mass. The street is icy, and as a result the coefficient of static friction between the car's tires and the street surface is μs = 0.2. Determine the steepest slope (in degrees relative to the horizontal) at which the car could remain in equilibrium if a. the brakes are applied to both its front and rear wheels; b. the brakes are applied to the front (lower) wheels only. Problem 9.5 1380 mm 532 mm 2370 mmarrow_forwardCan someone explain please with conversionsarrow_forwardCorrect Answer is written below. Detailed and complete fbd only please. I will upvote, thank you. 1: The assembly shown is composed of a rigid plank ABC, supported by hinge at A, spring at B and cable at C.The cable is attached to a frictionless pulley at D and rigidly supported at E. The cable is made of steel with E = 200,000MPa and cross-sectional area of 500 mm2. The details of pulley at D is shown. The pulley is supported by a pin, passingthough the pulley and attached to both cheeks. Note that E is directly above B.Given: H = 3 m; L1 = 2 m; L2 = 4 m; w = 12 kN/m; x:y = 3:4Spring Parameters:Wire diameter = 30 mmMean Radius = 90 mmNumber of turns = 12Modulus of Rigidity = 80 GPaAllowable stresses:Allowable shear stress of Pin at D = 85 MPaAllowable normal stress of cheek at D = 90MPaAllowable bearing stress of cheek at D = 110MPa1. Calculate the reaction of spring Band tension in cable at C.2. Calculate the vertical displacementat C and the required diameter ofpin at D.3.…arrow_forward
- Correct answer and complete fbd only. I will upvote. The compound shaft, composed of steel,aluminum, and bronze segments, carries the two torquesshown in the figure. If TC = 250 lb-ft, determine the maximumshear stress developed in each material (in ksi). The moduliof rigidity for steel, aluminum, and bronze are 12 x 106 psi, 4x 106 psi, and 6 x 106 psi, respectivelyarrow_forwardCan you explain the algebra steps that aren't shown but stated to be there, on how to get this equationarrow_forwardCorrect answer and complete fbd only. I will upvote. A flanged bolt coupling consists of two concentric rows of bolts. The inner row has 6 nos. of 16mm diameterbolts spaced evenly in a circle of 250mm in diameter. The outer row of has 10 nos. of 25 mm diameter bolts spaced evenly in a circle of 500mm in diameter. If the allowable shear stress on one bolt is 60 MPa, determine the torque capacity of the coupling. The Poisson’s ratio of the inner row of bolts is 0.2 while that of the outer row is 0.25 and the bolts are steel, E =200 GPa.arrow_forward
- Correct answer and complete fbd only. I will upvote. 10: The constant wall thickness of a steel tube with the cross sectionshown is 2 mm. If a 600-N-m torque is applied to the tube. Use G = 80 GPa forsteel.1. Find the shear stress (MPa) in the wall of the tube.2. Find the angle of twist, in degrees per meter of length.arrow_forwardCORRECT ANSWER WITH COMPLETE FBD ONLY. I WILL UPVOTE. A torque wrench is used to tighten the pipe shown.Dimensions: S1 = 400 mm; S2 = 250 mm; S3 = 100 mmModulus of Rigidity G = 78 GPa1. The diameter of the solid pipe is 20 mm. How much is themaximum force P (N) that can be applied based on theallowable shear stress of 60 MPa?2. For a hollow pipe with 50 mm outside diameter and is 6 mmthick, compute for the maximum force P (kN) that can beapplied such that the angle of twist at A does not exceed 5degrees.3. The torque applied to tighten the hollow pipe is 200 N-m.Given: Pipe outside diameter = 50 mm Pipe thickness = 6 mmSolve for the resulting maximum shear stress (MPa) in the pipe.arrow_forwardCorrect answer and complete fbd only. I will upvote. 6: The shaft carries a total torque T0 that is uniformly distributedover its length L. Determine the angle of twist (degrees) of the shaft in termsif T0 = 1.2 kN-m, L = 2 m, G = 80 GPa, and diameter = 120 mm.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Dimensional Analysis - in physics; Author: Jennifer Cash;https://www.youtube.com/watch?v=c_ZUnEUlTbM;License: Standard youtube license