
Concept explainers
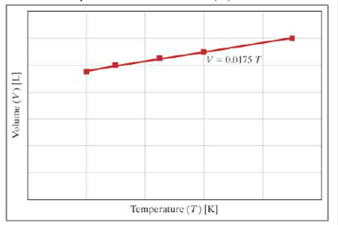
The graph shows the ideal gas law relationship (PV =nRT) between volume (V) and temperature (T).
- a. What are the units of the slope (0.0175)?
- b. If the tank has a pressure of 2.4 atmospheres and is filled with nitrogen (formula, N2; molecular weight, 28 grams per mole), what is the mass of gas in the tank in units of grams?
- c.
 If the tank is filled with 20 grams of oxygen (formula, o2; molecular weight, 32 grams per mole), what is the pressure of the tank (P) in units of atmospheres?
If the tank is filled with 20 grams of oxygen (formula, o2; molecular weight, 32 grams per mole), what is the pressure of the tank (P) in units of atmospheres?
a.
Find the slope units.
Answer to Problem 1ICA
The units of slope is
Explanation of Solution
Calculation:
Refer to the graph in the respective question. The expression is,
Rearrange the expression.
From the graph, the unit of volume (V) is L and temperature (T) is K. Therefore, the unit of slope (0.0175) is
Conclusion:
Thus, the units of slope is
b.
Find the mass of gas in terms of grams.
Answer to Problem 1ICA
The mass of nitrogen gas in terms of grams is 14.4 g.
Explanation of Solution
Given data:
Molecular weight (MW) of nitrogen is 28 grams per mole.
Pressure (P)is 2.4 atmospheres.
Formula used:
Consider the ideal gas law relationship.
Here,
Calculation:
Refer to the graph in the respective question. The expression is,
Modify equation (1) as follows.
Substitute
Substitute 2.4 atm for P and
Consider the general expression for mass in terms of molecular weight.
Substitute
Conclusion:
Thus, the mass of nitrogen gas in terms of grams is 14.4 g.
c.
Find the pressure of tank in terms of atmospheres.
Answer to Problem 1ICA
The pressure of tank in terms of atmospheres is 3.0 atm.
Explanation of Solution
Given data:
Molecular weight (MW) of oxygen is 32 grams per mole.
Mass of oxygen (m)is 20 grams.
Calculation:
Refer to the graph in the respective question.
Modify equation (2).
Substitute 20 g for
Modify equation (1).
Substitute
Substitute 0.625 mol for n and
Conclusion:
Thus, the pressure of tank in terms of atmospheres is 3.0 atm.
Want to see more full solutions like this?
Chapter 12 Solutions
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
- Correct answer is written below. Detailed and complete solution only with fbd. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forward
- Correct answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





