
- 13. It is difficult to bring the Internet to some remote parts of the world. This can be inexpensively done by installing antennas tethered to large helium balloons. To help analyze the situation, assume we have inflated a large spherical balloon. The pressure on the inside of the balloon is balanced by the elastic force exerted by the rubberized material. Since we are dealing with a gas in an enclosed space, the ideal gas law will be applicable.
PV=nRT
Where
P = pressure [atm]
V = volume [L]
n = quant ity of gas [moles]
R = ideal gas constant [0.08206 (atm L)/ (mol K)]
T = temperature [K]
If the temperature increases, the balloon will expand and/or the pressure will increase to maintain the equality. As it turns out, the increase in volume is the dominant effect, so we will treat the change in pressure as negligible.
The circumference of an inflated spherical balloon is measured at various temperatures; the resulting data are shown in the following graph.
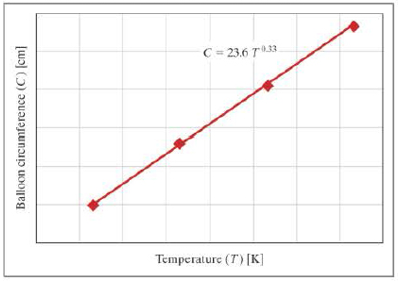
- a. What are the units of the constant 0.33?
- b. What are the units of the constant 23.6?
- c. What would the temperature of the balloon be if the circumference was 162 centimeters?
- d. If a circle with an area of 100 square centimeters is drawn on the balloon at 20 degrees Celsius, what would the area be at a temperature of 100 degrees Celsius?
- e. If the pressure inside the balloon is 1.2 atmospheres, how many moles of gas does it contain?
Trending nowThis is a popular solution!

Chapter 12 Solutions
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Additional Engineering Textbook Solutions
Vector Mechanics For Engineers
Mechanics of Materials (10th Edition)
Database Concepts (8th Edition)
Electric Circuits. (11th Edition)
Thermodynamics: An Engineering Approach
Starting Out with C++ from Control Structures to Objects (9th Edition)
- (read me)arrow_forward(read image)arrow_forwardQu. 13 What are the indices for the Direction 2 indicated by vector in the following sketch? Qu. 14 Determine the indices for the direction A and B shown in the following cubic unit cell. please show all work step by step from material engineeringarrow_forward
- The thin-walled open cross section shown is transmitting torque 7. The angle of twist ₁ per unit length of each leg can be determined separately using the equation 01 = 3Ti GLIC 3 where G is the shear modulus, ₁ is the angle of twist per unit length, T is torque, and L is the length of the median line. In this case, i = 1, 2, 3, and T; represents the torque in leg i. Assuming that the angle of twist per unit length for each leg is the same, show that T= Lic³ and Tmaz = G01 Cmax Consider a steel section with Tallow = 12.40 kpsi. C1 2 mm L1 20 mm C2 3 mm L2 30 mm C3 2 mm L3 25 mm Determine the torque transmitted by each leg and the torque transmitted by the entire section. The torque transmitted by the first leg is | N-m. The torque transmitted by the second leg is N-m. The torque transmitted by the third leg is N-m. The torque transmitted by the entire section is N-m.arrow_forwardPlease help, make sure it's to box out and make it clear what answers go where...arrow_forwardThe cylinder floats in the water and oil to the level shown. Determine the weight of the cylinder. (rho)o=910 kg/m^3arrow_forward
 Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning

