
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.2, Problem 2CYU
Potassium chloride has the same unit cell as NaCl. Using the ion sizes in Figure 7.11, calculate the density of KCl.
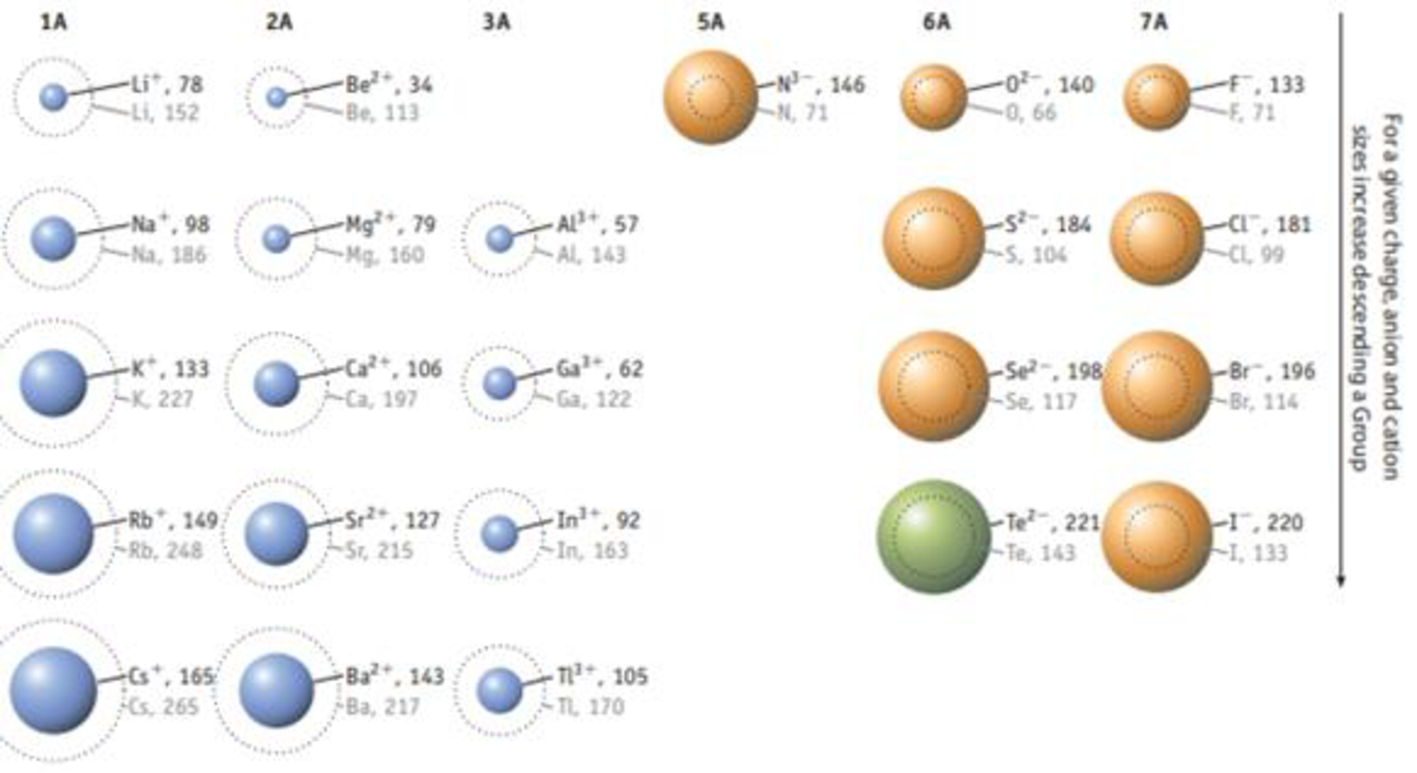
FIGURE 7.11 Relative sizes of some common ions. Rodii are given in picometers (1 pm 1 × 10‒12 m). (Data taken from J. Emsley, The Elements, Clarendon Press, Oxford, 1998, 3rd edition.)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Can you please help me solve these homework questions
An einstein is the amount of energy needed to dissociate 1 mole of a substance. If we have 0.58 moles, do we need 0.58 einsteins to dissociate that substance?
If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.Data: Energy of each photon: 0.7835x10-18 J.
Chapter 12 Solutions
Chemistry & Chemical Reactivity
Ch. 12.1 - (a) Determining an Atom Radius from Lattice...Ch. 12.1 - Prob. 1RCCh. 12.1 - Prob. 2RCCh. 12.2 - If an ionic solid has an fcc lattice of anions (X)...Ch. 12.2 - Potassium chloride has the same unit cell as NaCl....Ch. 12.2 - 1. The unit cell of silicon carbide. SiC is...Ch. 12.2 - If one edge of the silicon carbide unit cell is...Ch. 12.2 - Prob. 1QCh. 12.2 - Describe the unit cell of lithium (see Figure).Ch. 12.2 - Prob. 3Q
Ch. 12.2 - Prob. 4QCh. 12.3 - Prob. 1RCCh. 12.3 - Prob. 2RCCh. 12.3 - Prob. 3RCCh. 12.4 - Prob. 1RCCh. 12.5 - Prob. 1QCh. 12.5 - Prob. 2QCh. 12.5 - Prob. 3QCh. 12.5 - 1. Which of the following allotropes of carbon is...Ch. 12.5 - Prob. 2RCCh. 12.6 - Prob. 1RCCh. 12.6 - Suppose you wanted to cool 100. g of water from 20...Ch. 12.7 - Prob. 1RCCh. 12.7 - How many tin atoms are contained in the tetragonal...Ch. 12.7 - Prob. 2QCh. 12.7 - Prob. 3QCh. 12.7 - Prob. 4QCh. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - Prob. 3PSCh. 12 - Rutile, TiO2, crystallizes in a structure...Ch. 12 - Cuprite is a semiconductor. Oxide ions are at the...Ch. 12 - The mineral fluorite, which is composed of calcium...Ch. 12 - Calcium metal crystallizes in a face-centered...Ch. 12 - The density of copper metal is 8.95 g/cm3. If the...Ch. 12 - Potassium iodide has a face-centered cubic unit...Ch. 12 - A unit cell of cesium chloride is illustrated in...Ch. 12 - Predict the trend in lattice energy, from least...Ch. 12 - Prob. 12PSCh. 12 - To melt an ionic solid, energy must be supplied to...Ch. 12 - Which compound in each of the following pairs...Ch. 12 - Prob. 15PSCh. 12 - Prob. 16PSCh. 12 - Considering only the molecular orbitals formed by...Ch. 12 - Prob. 18PSCh. 12 - Prob. 19PSCh. 12 - Prob. 20PSCh. 12 - Prob. 21PSCh. 12 - Prob. 22PSCh. 12 - Prob. 23PSCh. 12 - Prob. 24PSCh. 12 - A diamond unit cell is shown here. Unit cell of...Ch. 12 - The structure of graphite is given in Figure...Ch. 12 - We have identified six types of solids (metallic,...Ch. 12 - Prob. 28PSCh. 12 - Classify each of the following materials as...Ch. 12 - Prob. 30PSCh. 12 - Benzene, C6H6, is an organic liquid that freezes...Ch. 12 - The specific heat capacity of silver is 0.235 J/g ...Ch. 12 - Prob. 33PSCh. 12 - Prob. 34PSCh. 12 - Prob. 35PSCh. 12 - If your air conditioner is more than several years...Ch. 12 - Sketch a phase diagram for O2 from the following...Ch. 12 - Tungsten crystallizes in the unit cell shown here....Ch. 12 - Silver crystallizes in a face-centered cubic unit...Ch. 12 - The unit cell shown here is for calcium carbide....Ch. 12 - The very dense metal iridium has a face-centered...Ch. 12 - Vanadium metal has a density of 6.11 g/cm3....Ch. 12 - Prob. 43GQCh. 12 - Prob. 44GQCh. 12 - Prob. 45GQCh. 12 - Consider the three types of cubic units cells. (a)...Ch. 12 - The solid-state structure of silicon is shown...Ch. 12 - The solid-state structure of silicon carbide is...Ch. 12 - Spinels are solids with the general formula AB2O4...Ch. 12 - Using the thermochemical data below and an...Ch. 12 - Prob. 51GQCh. 12 - Prob. 52GQCh. 12 - Prob. 53GQCh. 12 - Prob. 54GQCh. 12 - Prob. 55GQCh. 12 - Prob. 56GQCh. 12 - Like ZnS, lead(II) sulfide, PbS (commonly called...Ch. 12 - CaTiO3, a perovskite, has the structure below. (a)...Ch. 12 - Potassium bromide has the same lattice structure...Ch. 12 - Calculate the lattice energy of CaCl2 using a...Ch. 12 - Why is it not possible for a salt with the formula...Ch. 12 - Prob. 63SCQCh. 12 - Prob. 64SCQCh. 12 - Prob. 65SCQCh. 12 - Phase diagrams for materials that have allotropes...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- The quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardIf the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forward
- If the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY