
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.2, Problem 2Q
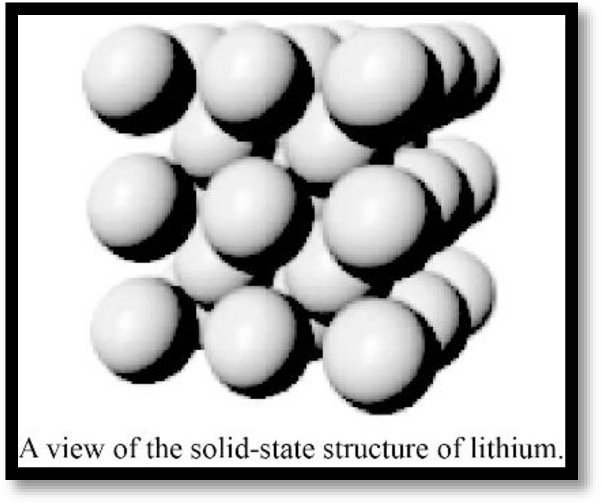
Describe the unit cell of lithium (see Figure).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
I need help with the following question
For CARS, which statement is not true regarding its advantages?
a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging.
b) Stronger signals than spontaneous Raman.
c) Suffers from fluorescence interference, because CARS signal is at high frequency.
d) Faster, more efficient imaging for real-time analysis.
e) Higher resolution than spontaneous Raman microscopy.
Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore
inorganic byproducts.
Incorrect, 5 attempts remaining
1. NaOCH3/CH3OH
2. Acidic workup
Select to Draw
O
Incorrect, 5 attempts remaining
The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or
intermediate structures and recount the number of carbon atoms in the parent chain of your structure.
OK
Chapter 12 Solutions
Chemistry & Chemical Reactivity
Ch. 12.1 - (a) Determining an Atom Radius from Lattice...Ch. 12.1 - Prob. 1RCCh. 12.1 - Prob. 2RCCh. 12.2 - If an ionic solid has an fcc lattice of anions (X)...Ch. 12.2 - Potassium chloride has the same unit cell as NaCl....Ch. 12.2 - 1. The unit cell of silicon carbide. SiC is...Ch. 12.2 - If one edge of the silicon carbide unit cell is...Ch. 12.2 - Prob. 1QCh. 12.2 - Describe the unit cell of lithium (see Figure).Ch. 12.2 - Prob. 3Q
Ch. 12.2 - Prob. 4QCh. 12.3 - Prob. 1RCCh. 12.3 - Prob. 2RCCh. 12.3 - Prob. 3RCCh. 12.4 - Prob. 1RCCh. 12.5 - Prob. 1QCh. 12.5 - Prob. 2QCh. 12.5 - Prob. 3QCh. 12.5 - 1. Which of the following allotropes of carbon is...Ch. 12.5 - Prob. 2RCCh. 12.6 - Prob. 1RCCh. 12.6 - Suppose you wanted to cool 100. g of water from 20...Ch. 12.7 - Prob. 1RCCh. 12.7 - How many tin atoms are contained in the tetragonal...Ch. 12.7 - Prob. 2QCh. 12.7 - Prob. 3QCh. 12.7 - Prob. 4QCh. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - Prob. 3PSCh. 12 - Rutile, TiO2, crystallizes in a structure...Ch. 12 - Cuprite is a semiconductor. Oxide ions are at the...Ch. 12 - The mineral fluorite, which is composed of calcium...Ch. 12 - Calcium metal crystallizes in a face-centered...Ch. 12 - The density of copper metal is 8.95 g/cm3. If the...Ch. 12 - Potassium iodide has a face-centered cubic unit...Ch. 12 - A unit cell of cesium chloride is illustrated in...Ch. 12 - Predict the trend in lattice energy, from least...Ch. 12 - Prob. 12PSCh. 12 - To melt an ionic solid, energy must be supplied to...Ch. 12 - Which compound in each of the following pairs...Ch. 12 - Prob. 15PSCh. 12 - Prob. 16PSCh. 12 - Considering only the molecular orbitals formed by...Ch. 12 - Prob. 18PSCh. 12 - Prob. 19PSCh. 12 - Prob. 20PSCh. 12 - Prob. 21PSCh. 12 - Prob. 22PSCh. 12 - Prob. 23PSCh. 12 - Prob. 24PSCh. 12 - A diamond unit cell is shown here. Unit cell of...Ch. 12 - The structure of graphite is given in Figure...Ch. 12 - We have identified six types of solids (metallic,...Ch. 12 - Prob. 28PSCh. 12 - Classify each of the following materials as...Ch. 12 - Prob. 30PSCh. 12 - Benzene, C6H6, is an organic liquid that freezes...Ch. 12 - The specific heat capacity of silver is 0.235 J/g ...Ch. 12 - Prob. 33PSCh. 12 - Prob. 34PSCh. 12 - Prob. 35PSCh. 12 - If your air conditioner is more than several years...Ch. 12 - Sketch a phase diagram for O2 from the following...Ch. 12 - Tungsten crystallizes in the unit cell shown here....Ch. 12 - Silver crystallizes in a face-centered cubic unit...Ch. 12 - The unit cell shown here is for calcium carbide....Ch. 12 - The very dense metal iridium has a face-centered...Ch. 12 - Vanadium metal has a density of 6.11 g/cm3....Ch. 12 - Prob. 43GQCh. 12 - Prob. 44GQCh. 12 - Prob. 45GQCh. 12 - Consider the three types of cubic units cells. (a)...Ch. 12 - The solid-state structure of silicon is shown...Ch. 12 - The solid-state structure of silicon carbide is...Ch. 12 - Spinels are solids with the general formula AB2O4...Ch. 12 - Using the thermochemical data below and an...Ch. 12 - Prob. 51GQCh. 12 - Prob. 52GQCh. 12 - Prob. 53GQCh. 12 - Prob. 54GQCh. 12 - Prob. 55GQCh. 12 - Prob. 56GQCh. 12 - Like ZnS, lead(II) sulfide, PbS (commonly called...Ch. 12 - CaTiO3, a perovskite, has the structure below. (a)...Ch. 12 - Potassium bromide has the same lattice structure...Ch. 12 - Calculate the lattice energy of CaCl2 using a...Ch. 12 - Why is it not possible for a salt with the formula...Ch. 12 - Prob. 63SCQCh. 12 - Prob. 64SCQCh. 12 - Prob. 65SCQCh. 12 - Phase diagrams for materials that have allotropes...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forwardCan you help me understand the CBC method on metal bridging by looking at this problem?arrow_forward
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY