
Study Guide And Selected Solutions Manual For Chemistry Format: Paperback
13th Edition
ISBN: 9780134553986
Author: Timberlake, Karen C
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.2, Problem 12.10PP
Classify each of the following alcohols as primary (1°), secondary (2°), or tertiary (3°):
a.
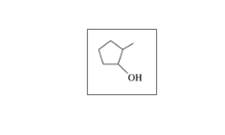
b.
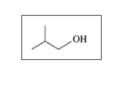
c.
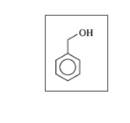
d.
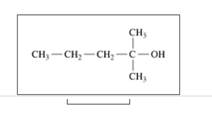
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part IV. Propose a plausible Structure w/ the following descriptions:
a) A 5-carbon hydrocarbon w/ a single peak in its proton decoupled
the DEPT-135 Spectrum shows a negative peak
C-NMR spectrum where
b) what cyclohexane dione isomer gives the largest no. Of 13C NMR signals?
c) C5H120 (5-carbon alcohol) w/ most deshielded carbon absent in any of its DEPT Spectiva
13C NMR is good for:
a) determining the molecular weight of
the compound
b) identifying certain functional groups.
c) determining the carbon skeleton, for
example methyl vs ethyl vs propyl groups
d) determining how many different kinds
of carbon are in the molecule
6
D
2. (1 pt) Limonene can be isolated by performing steam distillation of orange peel.
Could you have performed this experiment using hexane instead of water? Explain.
3. (2 pts) Using GCMS results, analyze and discuss the purity of the Limonene obtained
from the steam distillation of orange peel.
Chapter 12 Solutions
Study Guide And Selected Solutions Manual For Chemistry Format: Paperback
Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Prob. 12.3PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Give the common name for each of the following: a....Ch. 12.1 - Prob. 12.6PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Draw the condensed structural formula, or...Ch. 12.2 - Classify each of the following alcohols as primary...Ch. 12.2 - Classify each of the following alcohols as primary...
Ch. 12.2 - Prob. 12.11PPCh. 12.2 - Prob. 12.12PPCh. 12.2 - Give an explanation for each of the following...Ch. 12.2 - Give an explanation for each of the following...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Prob. 12.27PPCh. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Prob. 12.33PPCh. 12.4 - Prob. 12.34PPCh. 12.4 - Oxybenzone is an effective sunscreen whose...Ch. 12.4 - Avobenzone is a common ingredient in sunscreen....Ch. 12 - Prob. 12.37UTCCh. 12 - The compound frambinone has the taste of...Ch. 12 - A compound called resveratrol is an antioxidant,...Ch. 12 - A compound called cinnamaldehyde is found in...Ch. 12 - Prob. 12.41UTCCh. 12 - Prob. 12.42UTCCh. 12 - Prob. 12.43APPCh. 12 - Classify each of the following alcohols as primary...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.63CPCh. 12 - Draw the condensed structural formulas and give...Ch. 12 - A compound with the formula C4H8O is synthesized...Ch. 12 - A compound with the formula C5H10O oxidizes to...Ch. 12 - Compound A is a primary alcohol whose formula is...Ch. 12 - Compound X is a secondary alcohol whose formula is...Ch. 12 - Prob. 21CICh. 12 - Prob. 22CICh. 12 - Prob. 23CICh. 12 - Prob. 24CICh. 12 - Prob. 25CICh. 12 - lonone is a compound that gives violets their...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part III. Arrange the following carbons (in blue) in order of increasing chemical shift. HO B NH 2 A CIarrow_forward6. Choose the compound that will produce the spectrum below and assign the signals as carbonyl, aryl, or alkyl. 100 ō (ppm) 50 0 7. 200 150 Assign all of the protons on the spectrum below. 8. A B 4 E C 3 ō (ppm) 2 1 0 Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. OH 6 OH 3 2 1 0 4 ō (ppm)arrow_forwardIn the Thermo Fisher application note about wine analysis (Lesson 3), the following chromatogram was collected of nine components of wine. If peak 3 has a retention time of 3.15 minutes and a peak width of 0.070 minutes, and peak 4 has a retention time of 3.24 minutes and a peak width of 0.075 minutes, what is the resolution factor between the two peaks? [Hint: it will help to review Lesson 2 for this question.] MAU 300 200 T 34 5 100- 1 2 CO 6 7 8 9 0 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 4.0 4.2 4.4 4.6 4.8 5.0 5.2 Minutes 3.22 0.62 1.04 O 1.24arrow_forward
- The diagram shows two metals, A and B, which melt at 1000°C and 1400°C. State the weight percentage of the primary constituent (grains of C) that would be obtained by solidifying a 20% alloy of B. 1000°C a+L L+C 900°С 12 α a+C 45 1200 C L+y 140096 C+Y a+ß 800°C 700°C C+B 96 92 a+B 0 10 20 30 40 50 60 70 80 90 100 A % peso B Barrow_forward8. Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. 2 4 3 ō (ppm) OH 4 6 6 СОН 2 1 0arrow_forward7. Assign all of the protons on the spectrum below. A B 2 C E 2 1 3 6 4 3 2 1 0arrow_forward
- e. If (3R,4R)-3,4-dichloro-2,5-dimethylhexane and (3R,4S)-3,4-dichloro-2,5-dimethylhexane are in a solution at the same concentration, would this solution be expected to rotate plane polarized light (that is, be optically active)? Please provide your reasoning for your answer. [If you read this problem carefully, you will not need to draw out the structures to arrive at your answer...]arrow_forward1. How many neighbors does the proton that produces the multiplet below have? 2. 3. اللـ Draw a partial structure from the multiplet below. (The integration of the multiplet is 6) M Using the additivity constants found in appendix G of your lab manual, calculate the approximate chemical shifts of the protons indicated below. (Show your work!!!) B A Br SHarrow_forward1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°? To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide. kindly show me how to solve this long problem. Thanksarrow_forward
- 4. An 'H-NMR of a compound is acquired. The integration for signal A is 5692 and the integration for signal B is 25614. What is the simplest whole number ratio of protons for signals A and B? (Show your work!!!) 5. Assign the carbons in the NMR below as either carbonyl, aromatic, or alkyl. 200 150 100 50 ō (ppm) 1arrow_forwardSpeaking of composite materials, indicate the correct option:(A). Composite materials can only be: metal-polymer or polymer-polymer.(B). Composite materials can be made up of particles, but not fibers or sheets.(C). When the reinforcing particles are uniformly distributed in a composite material, there may be a greater tendency for it to have isotropic properties.(D). None of the above is correct.arrow_forwardIf we are talking about viscoelastic modulus or viscoelastic relaxation modulus in polymers, indicate the correct option.(A). It reports the variation of elastic behavior as a function of time.(B). It is only useful for defining its glass transition temperature.(C). It only allows us to define the polymer degradation temperature.(D). Neither option is correct.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License