
Study Guide And Selected Solutions Manual For Chemistry Format: Paperback
13th Edition
ISBN: 9780134553986
Author: Timberlake, Karen C
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.1, Problem 12.2PP
Give the IUPAC name for each of the following:
a.
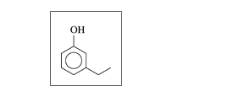
b.
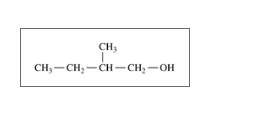
c.
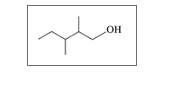
d.
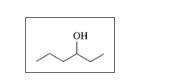
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
protons.
Calculate the mass (in grams) of H3AsO4 (MW=141.9416) needed to produce 3.125 x
1026
Please provide with answer, steps and explanation of ideas to solve.
Please provide with answer, steps and explanation of ideas to solve.
Chapter 12 Solutions
Study Guide And Selected Solutions Manual For Chemistry Format: Paperback
Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Prob. 12.3PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Give the common name for each of the following: a....Ch. 12.1 - Prob. 12.6PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Draw the condensed structural formula, or...Ch. 12.2 - Classify each of the following alcohols as primary...Ch. 12.2 - Classify each of the following alcohols as primary...
Ch. 12.2 - Prob. 12.11PPCh. 12.2 - Prob. 12.12PPCh. 12.2 - Give an explanation for each of the following...Ch. 12.2 - Give an explanation for each of the following...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Prob. 12.27PPCh. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Prob. 12.33PPCh. 12.4 - Prob. 12.34PPCh. 12.4 - Oxybenzone is an effective sunscreen whose...Ch. 12.4 - Avobenzone is a common ingredient in sunscreen....Ch. 12 - Prob. 12.37UTCCh. 12 - The compound frambinone has the taste of...Ch. 12 - A compound called resveratrol is an antioxidant,...Ch. 12 - A compound called cinnamaldehyde is found in...Ch. 12 - Prob. 12.41UTCCh. 12 - Prob. 12.42UTCCh. 12 - Prob. 12.43APPCh. 12 - Classify each of the following alcohols as primary...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.63CPCh. 12 - Draw the condensed structural formulas and give...Ch. 12 - A compound with the formula C4H8O is synthesized...Ch. 12 - A compound with the formula C5H10O oxidizes to...Ch. 12 - Compound A is a primary alcohol whose formula is...Ch. 12 - Compound X is a secondary alcohol whose formula is...Ch. 12 - Prob. 21CICh. 12 - Prob. 22CICh. 12 - Prob. 23CICh. 12 - Prob. 24CICh. 12 - Prob. 25CICh. 12 - lonone is a compound that gives violets their...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardUsing what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forwardDraw a mechanism for the formation of 2-bromovanillin using bromonium ion as the reactive electrophile.arrow_forward
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardIndicate whether the copper(II) acetate dimer, in its dihydrated form with the formula [(CH3COO)2Cu]2·2H2O, is a metal cluster, a cage compound, or neither.arrow_forwardPlease correct answer and don't use hand ratingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY