
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
12th Edition
ISBN: 9781119664635
Author: Solomons
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 7PP
Practice Problem 12.7
Provide retrosynthetic analyses and syntheses for each of the following alcohols, starting with appropriate alkyl or aryl halides.
(a)
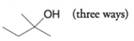
(b)
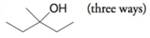
(c)
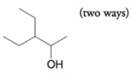
(d)
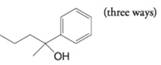
(e)
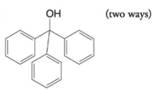
(f)
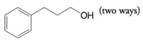
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A pdf file of your hand drawn, stepwise mechanisms for the reactions.
For each reaction in the assignment, you must write each mechanism
three times (there are 10 reactions, so 30 mechanisms). (A) do the work
on a tablet and save as a pdf., it is expected to write each mechanism
out and NOT copy and paste the mechanism after writing it just once.
Everything should be drawn out stepwise and every bond that is formed
and broken in the process of the reaction, and is expected to see all
relevant lone pair electrons and curved arrows.
Aldol:
NaOH
HO
H
Δ
NaOH
Δ
None
Draw structures corresponding to the following names and give IUPAC names for the
following compounds: (8 Point)
a)
b)
c)
CH3
CH2CH3
CH3CHCH2CH2CH
CH3
C=C
H3C
H
H2C=C=CHCH3
d)
CI
e) (3E,5Z)-2,6-Dimethyl-1,3,5,7-octatetraene
f) (Z)-4-bromo-3-methyl-3-penten-1-yne
g) cis-1-Bromo-2-ethylcyclopentane
h) (5R)-4,4,5-trichloro-3,3-dimethyldecane
Chapter 12 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Ch. 12 - Prob. 1PPCh. 12 - Prob. 2PPCh. 12 - Prob. 3PPCh. 12 - PRACTICE PROBLEM 12.4 Predict the products of the...Ch. 12 - Prob. 5PPCh. 12 - Prob. 6PPCh. 12 - Practice Problem 12.7
Provide retrosynthetic...Ch. 12 - Prob. 8PPCh. 12 - What products would you expect from the reaction...Ch. 12 - What products would you expect from the reaction...
Ch. 12 - What product (or products) would be formed from...Ch. 12 - Prob. 12PCh. 12 - 12.13 Write reaction conditions and the product...Ch. 12 - Prob. 14PCh. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the major organic product from each of the...Ch. 12 - Synthesize each of the following compounds from...Ch. 12 - Prob. 20PCh. 12 - 21. Write a mechanism for the following reaction....Ch. 12 - Prob. 22PCh. 12 - 23. What organic products A-H would you expect...Ch. 12 - Prob. 24PCh. 12 - Show how 1-pentanol could be transformed into each...Ch. 12 - Provide the reagents needed to accomplish...Ch. 12 - Prob. 27PCh. 12 - For each of the following alcohols, write a...Ch. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Predict the major organic product from each of the...Ch. 12 - 34. Synthesize the following compound using...Ch. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - 37. Explain how and IR spectroscopy could be used...Ch. 12 - 38. An unknown X shows a broad absorption band in...Ch. 12 - Prob. 39PCh. 12 - The problem below is directed toward devising a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
4. Two of these organ system bear the major responsibility for ensuring homeostasis of the internal environment...
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Examine the graph in Figure 6.3. Note that the growth rate increases slowly until the optimum is reached and th...
Microbiology with Diseases by Body System (5th Edition)
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
17.25 You are asked to prepare a pH = 3.00 buffer solution starting from 1.25 L of a 1.00 M solution of hydrofl...
Chemistry: The Central Science (14th Edition)
When you rub your cold hands together, the friction between them results in heat that warms your hands. Why doe...
Anatomy & Physiology (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a Newman projection from carbon 3 to carbon 2 in the highest energy conformation for the following molecule. What is this conformation called? What kind of strain is present? Brarrow_forwardWhich of the following dienophiles is most reactive in a Diels-Alder reaction: Please explain why the correct answer to this question is option 5. Please provide a detailed explanation.arrow_forwardWhich of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forward
- Draw the enantiomer and diastereomers of the following molecule. Label each type of stereoisomers. Label each chiral center as R or S. HOarrow_forwardWhich diene and dienophile would you choose to synthesize the following compound? Please provide a detailed explanation. Please include a drawing showing the mechanism of the synthesis. Please also explain why it is the correct diene and dienophile.arrow_forwardUsing the sketcher below, draw the structure of N-ethyldecylamine. Answer: 0 ୨୫) . 始 {n [ ]t ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY