
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 6PS
Rutile, TiO2, crystallizes in a structure characteristic of many other ionic compounds How many formula units of TiO2 are in the unit cell illustrated here? (The oxide ions marked by an x are wholly within the cell; the others are in the cell faces.)
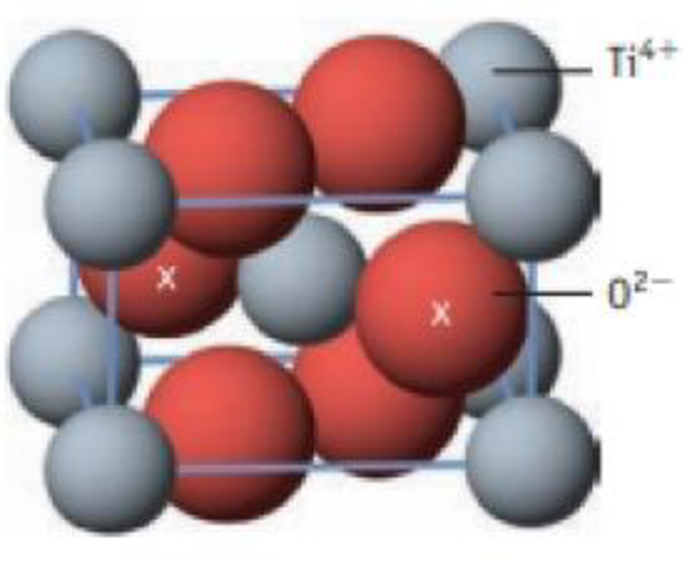
Unit cell for ruffle
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Chapter 12 Solutions
Chemistry & Chemical Reactivity
Ch. 12.1 - (a) Determining an Atom Radius from Lattice...Ch. 12.2 - If an ionic solid has an fcc lattice of anions (X)...Ch. 12.2 - Potassium chloride has the same unit cell as NaCl....Ch. 12.6 - Prob. 1.1ACPCh. 12.6 - Describe the unit cell of lithium (see Figure).Ch. 12.6 - Prob. 1.3ACPCh. 12.6 - Prob. 1.4ACPCh. 12.6 - Prob. 2.1ACPCh. 12.6 - Prob. 2.2ACPCh. 12.6 - Prob. 2.3ACP
Ch. 12.6 - How many tin atoms are contained in the tetragonal...Ch. 12.6 - Prob. 3.2ACPCh. 12.6 - Prob. 3.3ACPCh. 12.6 - Prob. 3.4ACPCh. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - A portion of the crystalline lattice for potassium...Ch. 12 - The unit cell of silicon carbide, SiC, is...Ch. 12 - Prob. 5PSCh. 12 - Rutile, TiO2, crystallizes in a structure...Ch. 12 - Cuprite is a semiconductor. Oxide ions are at the...Ch. 12 - The mineral fluorite, which is composed of calcium...Ch. 12 - Calcium metal crystallizes in a face-centered...Ch. 12 - The density of copper metal is 8.95 g/cm3. If the...Ch. 12 - Potassium iodide has a face-centered cubic unit...Ch. 12 - A unit cell of cesium chloride is illustrated in...Ch. 12 - Predict the trend in lattice energy, from least...Ch. 12 - Prob. 14PSCh. 12 - To melt an ionic solid, energy must be supplied to...Ch. 12 - Which compound in each of the following pairs...Ch. 12 - Prob. 17PSCh. 12 - Prob. 18PSCh. 12 - Considering only the molecular orbitals formed by...Ch. 12 - Prob. 20PSCh. 12 - Prob. 21PSCh. 12 - Prob. 22PSCh. 12 - Prob. 23PSCh. 12 - Prob. 24PSCh. 12 - Prob. 25PSCh. 12 - Prob. 26PSCh. 12 - Prob. 27PSCh. 12 - Prob. 28PSCh. 12 - A diamond unit cell is shown here. Unit cell of...Ch. 12 - The structure of graphite is given in Figure...Ch. 12 - We have identified six types of solids (metallic,...Ch. 12 - Prob. 32PSCh. 12 - Classify each of the following materials as...Ch. 12 - Prob. 34PSCh. 12 - Benzene, C6H6, is an organic liquid that freezes...Ch. 12 - The specific heat capacity of silver is 0.235 J/g ...Ch. 12 - Prob. 37PSCh. 12 - Prob. 38PSCh. 12 - Prob. 39PSCh. 12 - If your air conditioner is more than several years...Ch. 12 - Sketch a phase diagram for O2 from the following...Ch. 12 - Tungsten crystallizes in the unit cell shown here....Ch. 12 - Silver crystallizes in a face-centered cubic unit...Ch. 12 - The unit cell shown here is for calcium carbide....Ch. 12 - The very dense metal iridium has a face-centered...Ch. 12 - Vanadium metal has a density of 6.11 g/cm3....Ch. 12 - Prob. 47GQCh. 12 - Prob. 48GQCh. 12 - Prob. 49GQCh. 12 - Consider the three types of cubic units cells. (a)...Ch. 12 - The solid-state structure of silicon is shown...Ch. 12 - The solid-state structure of silicon carbide is...Ch. 12 - Spinels are solids with the general formula AB2O4...Ch. 12 - Using the thermochemical data below and an...Ch. 12 - Prob. 55GQCh. 12 - Prob. 56GQCh. 12 - Prob. 57GQCh. 12 - Prob. 58GQCh. 12 - Prob. 59GQCh. 12 - Prob. 60GQCh. 12 - Like ZnS, lead(II) sulfide, PbS (commonly called...Ch. 12 - CaTiO3, a perovskite, has the structure below. (a)...Ch. 12 - Potassium bromide has the same lattice structure...Ch. 12 - Calculate the lattice energy of CaCl2 using a...Ch. 12 - Why is it not possible for a salt with the formula...Ch. 12 - Prob. 67SCQCh. 12 - Prob. 68SCQCh. 12 - Prob. 69SCQCh. 12 - Phase diagrams for materials that have allotropes...
Additional Science Textbook Solutions
Find more solutions based on key concepts
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer the questions in the photos and please revise any wrong answers. Thank youarrow_forward(Please be sure that 7 carbons are available in the structure )Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10.arrow_forward-lease help me answer the questions in the photo.arrow_forward
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
- Help me understand this by showing step by step solution.arrow_forwardscratch paper, and the integrated rate table provided in class. our scratch work for this test. Content attribution 3/40 FEEDBACK QUESTION 3 - 4 POINTS Complete the equation that relates the rate of consumption of H+ and the rate of formation of Br2 for the given reaction. 5Br (aq) + BrO3 (aq) + 6H (aq) →3Br2(aq) + 3H2O(l) • Your answers should be whole numbers or fractions without any decimal places. Provide your answer below: Search 尚 5 fn 40 * 00 99+ 2 9 144 a [arrow_forward(a) Write down the structure of EDTA molecule and show the complex structure with Pb2+ . (b) When do you need to perform back titration? (c) Ni2+ can be analyzed by a back titration using standard Zn2+ at pH 5.5 with xylenol orange indicator. A solution containing 25.00 mL of Ni2+ in dilute HCl is treated with 25.00 mL of 0.05283 M Na2EDTA. The solution is neutralized with NaOH, and the pH is adjusted to 5.5 with acetate buffer. The solution turns yellow when a few drops of indicator are added. Titration with 0.02299 M Zn2+ requires 17.61 mL to reach the red end point. What is the molarity of Ni2+ in the unknown?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY