
Concept explainers
The solid-state structure of silicon is shown below.
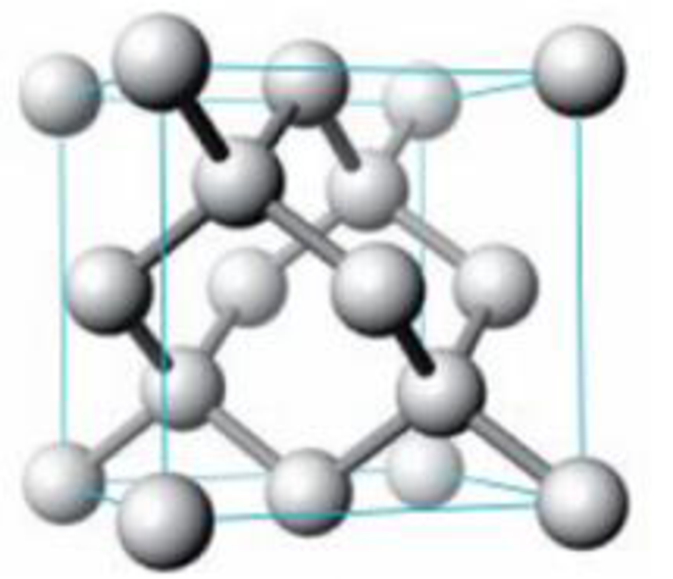
Unit cell for silicon
- (a) Describe this crystal as pc, bcc, or fcc.
- (b) What type of holes are occupied in the lattice?
- (c) How many Si atoms are there per unit cell?
- (d) Calculate the density of silicon in g/cm3 (given that the cube edge has a length of 543.1 pm).
- (e) Estimate the radius of the silicon atom. (Note: The Si atoms on the edges do not touch one another.)
(a)
Interpretation:
Given silicon crystal has to be described for PC, BCC or FCC.
Concept introduction:
- An ionic radii are the radius of an atom's ion in ionic crystals structure.
- An ionic solid is made up cations and anions held together by electrostatic forces in a rigid array or lattice.
- Positive charge ions are cations and negative charge ions are anions.
- Lattice Energy is mainly depends on the charge on the ion and radius or size of the ion.
- Ionic radius increases from top to bottom on the periodic table.
Ionic radius decreases from left to right the periodic table.
- The density of the unit cell:
- The face-centered cubic system:
It has lattice points on the faces of the cube, that each gives exactly one half contributions, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell
Answer to Problem 51GQ
The types of the lattice is face center cubic crystal
Explanation of Solution
The types of the lattice is face center cubic crystal, because It has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell
(b)
Interpretation:
The types of holes that are occupied in the lattice has to be determined.
Concept introduction:
- An ionic radii are the radius of an atom's ion in ionic crystals structure.
- An ionic solid is made up cations and anions held together by electrostatic forces in a rigid array or lattice.
- Positive charge ions are cations and negative charge ions are anions.
- Lattice Energy is mainly depends on the charge on the ion and radius or size of the ion.
- Ionic radius increases from top to bottom on the periodic table.
Ionic radius decreases from left to right the periodic table.
- The density of the unit cell:
- The face-centered cubic system:
It has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell
Answer to Problem 51GQ
Silicon atoms are located in one half of the tetrahedral holes
Explanation of Solution
Silicon atoms are located in one half of the tetrahedral holes.
(c)
Interpretation:
Number of silicon atoms per unit cell has to be determined.
Concept introduction:
- An ionic radii are the radius of an atom's ion in ionic crystals structure.
- An ionic solid is made up cations and anions held together by electrostatic forces in a rigid array or lattice.
- Positive charge ions are cations and negative charge ions are anions.
- Lattice Energy is mainly depends on the charge on the ion and radius or size of the ion.
- Ionic radius increases from top to bottom on the periodic table.
Ionic radius decreases from left to right the periodic table.
- The density of the unit cell:
- The face-centered cubic system:
It has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell
Answer to Problem 51GQ
Totally eight atoms in the unit cell
Explanation of Solution
Silicon atoms are located in one half of the tetrahedral holes.
(d)
Interpretation:
The density of the silicon atom has to be identified.
Concept introduction:
- An ionic radii are the radius of an atom's ion in ionic crystals structure.
- An ionic solid is made up cations and anions held together by electrostatic forces in a rigid array or lattice.
- Positive charge ions are cations and negative charge ions are anions.
- Lattice Energy is mainly depends on the charge on the ion and radius or size of the ion.
- Ionic radius increases from top to bottom on the periodic table.
Ionic radius decreases from left to right the periodic table.
- The density of the unit cell:
- The face-centered cubic system:
It has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell
Answer to Problem 51GQ
Density is
Explanation of Solution
The density of the silicon atom is given below,
(e)
Interpretation:
The radius of the silicon atom has to be identified.
Concept introduction:
- An ionic radii are the radius of an atom's ion in ionic crystals structure.
- An ionic solid is made up cations and anions held together by electrostatic forces in a rigid array or lattice.
- Positive charge ions are cations and negative charge ions are anions.
- Lattice Energy is mainly depends on the charge on the ion and radius or size of the ion.
- Ionic radius increases from top to bottom on the periodic table.
Ionic radius decreases from left to right the periodic table.
- The density of the unit cell:
- The face-centered cubic system:
It has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell
Answer to Problem 51GQ
Radius is
Explanation of Solution
The radius of silicon atom can be calculated as,
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry & Chemical Reactivity
Additional Science Textbook Solutions
Biology: Life on Earth with Physiology (11th Edition)
General, Organic, and Biological Chemistry - 4th edition
Genetics: From Genes to Genomes
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Biology: Life on Earth (11th Edition)
- A DEPT NMR spectrum is shown for a molecule with the molecular formula of C5H12O. Draw the structure that best fits this data. 200 180 160 140 120 100 一盆 00 40 8- 20 ppm 0 Qarrow_forwardDon't used hand raitingarrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forward
- Don't used hand raitingarrow_forwardS Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H H = HIN: H C. :0 H /\ H H Click and drag to start drawing a structure. ×arrow_forwardPlease help me figure out these calculation and what should be plotted. These are notes for my chemistry class.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning



