
Concept explainers
(a)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
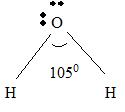
For H2 O
Part A Step 1 →
Sum(total atoms)
Step 2 →
Pair of electrons =
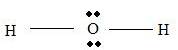
Electron pairs for bonds =
Step 3 → Remaining electron pairs = 2
Part B Step 1 → Lewis structure
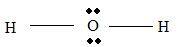
Step 2 → 4 electron pairs around
Step 3 → But around the
No: of bonding pairs =
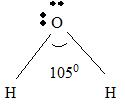
No: of non- bonding pairs =
Step 4 → Name of the structure = V-shaped or Bend.
(b)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
For PH3 Part A Step 1 →
Sum
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
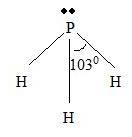
Part B Step 1 → Lewis structure
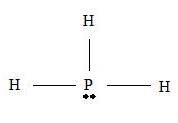
Step 2 →
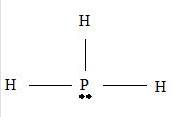
Step 3 → But around the
No: of non- bonding pairs = 1
Step 4 → Name of the structure = Trigonal pyramid.
(c)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
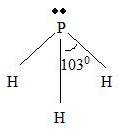
Explanation of Solution
CBr4 Part A. Step 1 →
Sum = 32
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
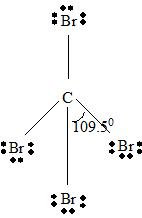
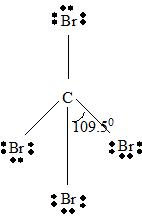
Part B Step 1 → Lewis structure
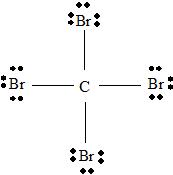
Step 2 →
Step 3 → But around the
No: of non- bonding pairs =
Step 4 → Name of the structure = Tetrahedral.
(d)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
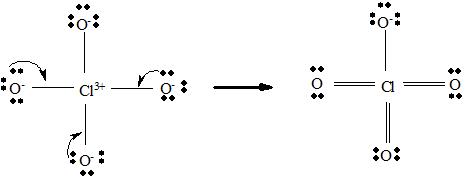
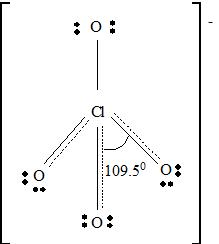
For ClO4 -
Part A. Step 1 →
Sum =
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
Part B Step 1 → Lewis structure
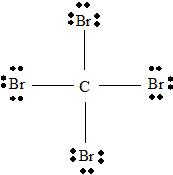
Step 2 →
Step 3 → But around the
No: of non- bonding pairs =
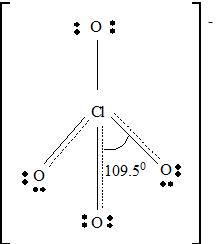
Step 4 → Name of the structure = Tetrahedral
But considering resonance, the exact position of three p bonds cannot be determined exactly. It provides a p cloud over the molecule. So that a resonance hybrid determines the exact structure of ClO4 - molecule.
(e)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
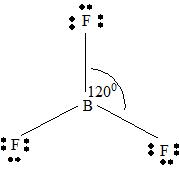
BF3
Part A. Step 1 →
Sum =
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
Part B Step 1 → Lewis structure
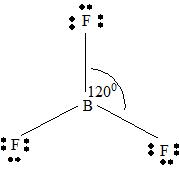
Step 2 →
Step 3 → Also around the
No: of bonding pairs =
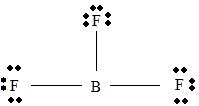
No: of non- bonding pairs =
Step 4 → Name of the structure = Trigonal planar.
(f)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
BeF2
Part A. Step 1 →
Sum =
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
Part B Step 1 → Lewis structure
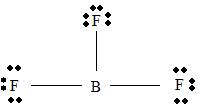
Step 2 →
Step 3 → Also around the
No: of non- bonding pairs =
Step 4 → Name of the structure = Linear
 .
.
Want to see more full solutions like this?
Chapter 12 Solutions
EBK INTRODUCTORY CHEMISTRY
- Anhydrous MgSO4 is used to:1. Form a salt with the compound dissolved in the solution2. Remove water from a solution3. Neutralize a solutionarrow_forwardDistillation under reduced pressure or vacuum consists of:1. Achieving distillation under anhydrous conditions.2. Causing a decrease in the distillation rate.3. Decreasing the pressure to lower the boiling point of the compound to be distilled.arrow_forwardAt the end of the silica gel production process, color changes occur during drying. Explain these color changes.arrow_forward
- If CoCl2/H2O is dissolved in a mixture of H2O and concentrated HCl in a test tube, the tube is gently heated over a flame to approximately 80°C and then cooled externally. Explain the color changes that occur.arrow_forwardWhen producing silica gel, color changes occur at the end of the drying process. Explain these color changes.arrow_forwardDesign experiments in UV-Vis to figure the optimal mole ratio of copper (1:1, 2:1, 3:1 and etc)versus ethambutol using all necessary chemicals including dihydrochloride and copper nitrate hemipentahydrate and sodium hydroxide. Show how UV-Vis absorbance and maximum wavelength would change in responsearrow_forward
- Correct each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO—CH,—C—CH,—OH X 5 2 2 2 HO–CH,—CH,—C—CH,—OH Explanation Check Center Accessi ©2025 on 5 Carrow_forwardMake the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).arrow_forwardH CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forward
- Homework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





