
Concept explainers
Give the IUPAC name for each compound.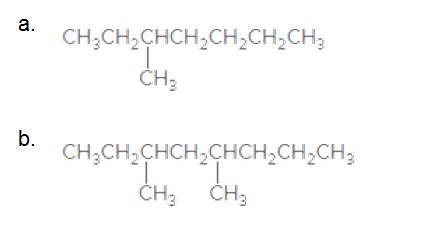
c.
e.
(a)
Interpretation:
The IUPAC nomenclature of alkanes having only one branched-chain needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
3-Methylheptane
Explanation of Solution
The longest carbon chain has 7 carbon.
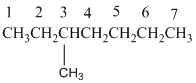
And there is a methyl group in the no 3 carbon. As the branch group has only one CH3, it is called 'Methyl'.
So, following by the above mention rule, the name of the alkane is 3 (the position of the branch) + Methyl (the name of the branch)+hept ( 7 carbon in main chain)+ "-ane"= 3-Methylheptane
(b)
Interpretation:
The IUPAC nomenclature of alkanes having 2 same brunches needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
3,5-dimethyloctane
Explanation of Solution
The longest carbon chain has 8 carbon.
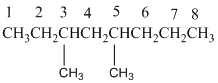
And there are two methyl groups in the no. 3 and no. 5 carbons. As the branch group has only one CH3, it is called 'Methyl'.
So, following by the above mention rule, the name of the alkane is 3,5 (the position of the branch) + dimethyl (the name of the branch, 'di' as two methyl are present)+ oct( 8 carbon in main chain)+ "-ane"= 3,5-dimethyloctane.
(c)
Interpretation:
The IUPAC nomenclature of Alkanes having 2 same brunches in same carbon needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
3,3-diethylhexane
Explanation of Solution
The longest carbon chain has 8 carbon.
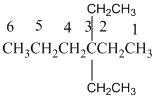
And there are two ethyl groups in the no. 3 carbon. As the branch groups have CH2CH3, it is called 'ethyl'.
So, following by the above mention rule, the name of the alkane is 3,3 (the position of the branch) + diethyl (the name of the branch, 'di' as two ethyl are present)+ hex( 6 carbon in main chain)+ "-ane"= 3,3-diethylhexane.
(d)
Interpretation:
The IUPAC nomenclature of Alkanes having skeletal structure needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
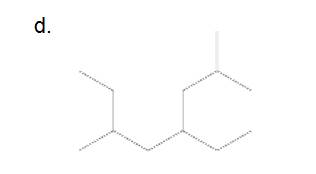
4-ethyl-2,6-dimethyloctane
Explanation of Solution
The longest carbon chain has 8 carbon.
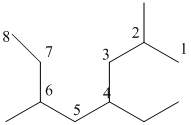
And there are two methyl groups in the no. 2 and 6 carbon, one ethyl group in no. 4carbon. As the branch groups have CH3and CH2CH3, they are called 'methyl' and 'ethyl' simultaneously.
So, following by the above mention rule, the name of the alkane is 4 (the position of the branch having alphabetically order 1st) +ethyl (the name of the branch) + 2,6 (position of 2nd brunch) + dimethyl( name of the brunch and 'di' as two methyl are present)+ oct ( 8 carbon in main chain)+ "-ane"= 4-ethyl-2,6-dimethyloctane
(e)
Interpretation:
The IUPAC nomenclature of alkanes having different brunches in different position needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
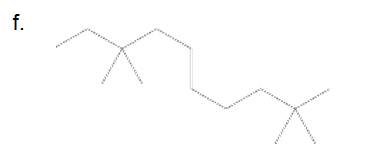
6-ethyl-2-methyloctane
Explanation of Solution
The longest carbon chain has 8 carbon.
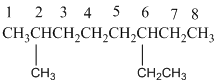
And there is one methyl group in the no. 2 carbon, one ethyl group in no. 6 carbon. As the branch groups have CH3and CH2CH3, they are called 'methyl' and 'ethyl' simultaneously.
So, following by the above mention rule, the name of the alkane is 6 (the position of the branch having alphabetically order 1st) + ethyl (the name of the branch) + 2 (position of 2nd brunch) + methyl( name of the brunch )+ oct ( 8 carbon in main chain)+ "-ane"= 6-ethyl-2-methyloctane
(f)
Interpretation:
The IUPAC nomenclature of Alkanes having lots of groups present in different position needs to be determined.
Concept Introduction:
Every organic compound has its own unique name which is followed by the IUPAC (International Union of Pure and Applied Chemistry). For alkane also, there is a particular rule for IUPAC nomenclature. The rules are −
- The name has to end with suffix "−ane" for all alkanes.
- The longest continuous carbon chain that contains the functional group will be treated as the main chain.
- Number the carbons in the longest carbon chain. As per as the no of carbon present in the longest chain, "Pent (for 5)", "Hept (for 6)" this word will be added before "ane".
- The branched groups present in the longest carbon chain beside the main chain should be named by the number of carbon atoms present in the branched group. These groups will end with "-yl" (e.g.- ethyl, methyl) at their end.
- The position of the group on the main carbon chain and it should be mentioned (e.g. 2,3 -).
- Numbering of the main chain should start from the side having smallest distance with most priority brunch (priority depends on alphabetical order of the brunch).
- The brunched groups should be listed before the name of the main carbon chain in alphabetical order (ignoring prefix like di/tri).
- Combine the elements of the name into a single word in the following order:
- Branched groups in alphabetical order (ignoring prefixes).
- Prefix of main chain
- End with "-ane" suffix.
Answer to Problem 45P
3,3,9,9-tetramethylundecane
Explanation of Solution
The longest carbon chain has 8 carbon.
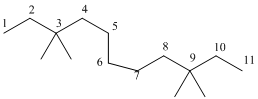
And there are four methyl groups in the no. 3 and 9 carbon. As the branch groups have CH3, it is called 'methyl'.
So, following by the above mention rule, the name of the alkane is 3,3,9,9 (the position of the branch) + tetramethyl ( name of the brunch and 'tetra' as four methyl are present)+ undacane ( 11 carbon in main chain)+ "-ane"= 3,3,9,9-tetramethylundecane.
Want to see more full solutions like this?
Chapter 12 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- In one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.arrow_forwardIn a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.arrow_forwardIf the current voltage is n = 0.14 V, indicate which of the 2 voltage formulas of the ley of Tafel must be applied i a a) == exp (1-B). xp[(1 - ß³): Fn Fn a b) == exp B RT RTarrow_forward
- If the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forward
- With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forwardApply the NANSTE law to the MnO4- + 8H+ + 5e- ⇄ Mn2+ + 4H2Oarrow_forwardIn the Nernst Law, how much is RT / F?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





