
(a)
Interpretation:
The
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called enantiomers
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer
(b)
Interpretation:
The alkyl halide which will be obtained in greater yield from the given reaction has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
(c)
Interpretation:
The alkyl halide which will be obtained in greater yield from the given reaction has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-super imposable mirror image is called enantiomers.
Diastereomers: A compound which is non-super imposable and non-mirror image is called enantiomers
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Bromination of
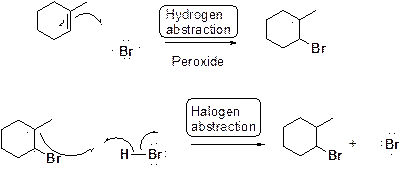
(d)
Interpretation:
The alkyl halide which will be obtained in greater yield from the given reaction has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-super imposable mirror image is called enantiomers.
Diastereomers: A compound which is non-super imposable and non-mirror image is called enantiomers
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Bromination of alkene in presence of peroxide:
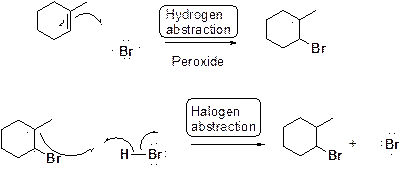
(e)
Interpretation:
The alkyl halide which will be obtained in greater yield from the given reaction has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-super imposable mirror image is called enantiomers.
Diastereomers: A compound which is non-super imposable and non-mirror image is called enantiomers
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Bromination of alkene in presence of peroxide:
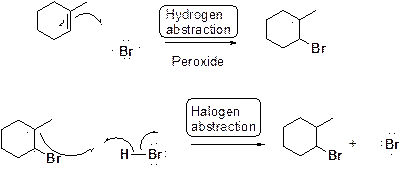
(f)
Interpretation:
The alkyl halide which will be obtained in greater yield from the given reaction has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-super imposable mirror image is called enantiomers.
Diastereomers: A compound which is non-super imposable and non-mirror image is called enantiomers
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Bromination of alkene in presence of peroxide:
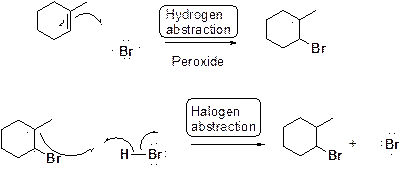
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- Steps and explanation.arrow_forwardProvide steps and explanation please.arrow_forwardDraw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forward
- Assign these protonarrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


