
Concept explainers
12-27 Explain why each name is incorrect and then write a correct name.
- 2-Ethyl-l-propene
- 5-lsopropylcyclohexene
- 4-Methyl-4-hexene
- 2-sec-Butyl-l-butene
- 6,6-Dimethylcyclohexene
- 2-Ethyl-2-hexene
(a)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P
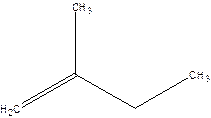
2-Methyl-but-1-ene.
Explanation of Solution
2-Ethyl-1-propene.
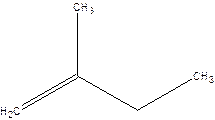
Error- longest chain not correctly located.
Correct name-
2-Methyl-but-1-ene.
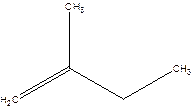
(b)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P
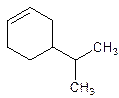
4-Isopropylcyclohexene.
Explanation of Solution
5-Isopropylcyclohexene.
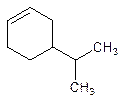
Error- position of the substituent not correctly mentioned.
Correct name-
4-Isopropylcyclohexene.
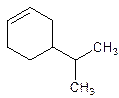
(c)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P

3-Methyl-2-hexene.
Explanation of Solution
4-Methyl-4-hexene.
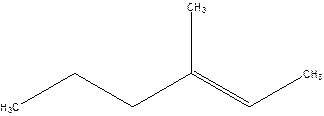
Error- position of the double bond not correctly mentioned.
Correct name-
3-Methyl-2-hexene.
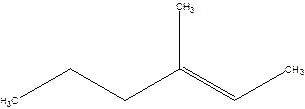
(d)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P
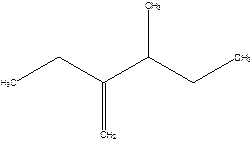
2-Ethyl-3-methyl -1-pentene.
Explanation of Solution
2-sec-butyl-1-butene.
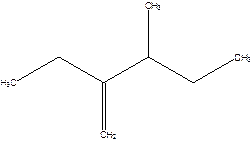
Error- longest chain not correctly determined.
Correct name-
2-Ethyl-3-methyl -1-pentene.
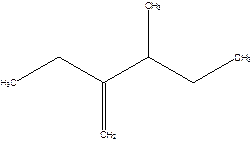
(e)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P
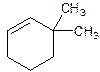
3,3-Dimethylcyclohexene.
Explanation of Solution
6,6-Dimethylcyclohexene.
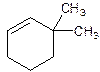
Error- position of substituents not correctly determined.
Correct name-
3,3-Dimethylcyclohexene.
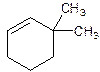
(f)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P

3-Methyl-3-Heptene.
Explanation of Solution
2-Ethyl-2-hexene.

Error- longest chain not correctly determined.
Correct name-
3-Methyl-3-Heptene.

Want to see more full solutions like this?
Chapter 12 Solutions
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
- AN IR spectrum, a 13 CMR spectrum, and a 1 HMR spectrum were obtained for an unknown structure with a molecular formula of C9H10. Draw the structure of this compound.arrow_forwardAN IR spectrum, a 13 CMR spectrum, and a 1 HMR spectrum were obtained for an unknown structure with a molecular formula of C9H10. Draw the structure of this compound.arrow_forward(a) What is the hybridization of the carbon in the methyl cation (CH3*) and in the methyl anion (CH3¯)? (b) What is the approximate H-C-H bond angle in the methyl cation and in the methyl anion?arrow_forward
- Q8: Draw the resonance structures for the following molecule. Show the curved arrows (how you derive each resonance structure). Circle the major resonance contributor.arrow_forwardQ4: Draw the Lewis structures for the cyanate ion (OCN) and the fulminate ion (CNO). Draw all possible resonance structures for each. Determine which form for each is the major resonance contributor.arrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. CH3 N CH3 HÖ: H3C CI: ::arrow_forward
- Q3: Draw the Lewis structures for nitromethane (CH3NO2) and methyl nitrite (CH3ONO). Draw at least two resonance forms for each. Determine which form for each is the major resonance contributor.arrow_forwardQ1: Draw a valid Lewis structures for the following molecules. Include appropriate charges and lone pair electrons. If there is more than one Lewis structure available, draw the best structure. NH3 Sulfate Boron tetrahydride. C3H8 (linear isomer) OCN NO3 CH3CN SO2Cl2 CH3OH2*arrow_forwardQ2: Draw all applicable resonance forms for the acetate ion CH3COO. Clearly show all lone pairs, charges, and arrow formalism.arrow_forward
- Please correct answer and don't used hand raitingarrow_forward9. The following reaction, which proceeds via the SN1/E1 mechanisms, gives three alkene products (A, B, C) as well as an ether (D). (a) Show how each product arises mechanistically. (b) For the alkenes, determine the major product and justify your answer. (c) What clues in the reaction as shown suggest that this reaction does not go by the SN2/E2 mechanism route? (CH3)2CH-CH-CH3 CH3OH 1 Bl CH3OH ⑧· (CH3)2 CH-CH=CH2 heat H ⑥③ (CH3)2 C = C = CH3 © СнЗ-С-Снаснз сна (CH 3 ) 2 C H G H CH 3 оснзarrow_forwardPlease Don't used hand raitingarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




