
Concept explainers
Explain why each name is incorrect and then write a correct name. Strategy: First draw the structural formula suggested by the incorrect name. Then identify the error, for example, failure to locate the longest chain that contains the
(a) 1-Methylpropene (b) 3-Pentene
(c) 2-Methylcyclohexene (d) 3,3-Dimethylpentene
(e) 4-Hexyne
(f) 2-lsopropyl-2-butene
(a)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
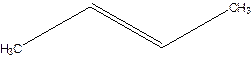
But-2-ene.
Explanation of Solution
1-Methylpropene.
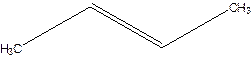
Error- longest chain not correctly located.
Correct name-
But-2-ene.
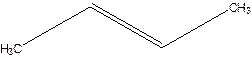
But-2-ene.
(b)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
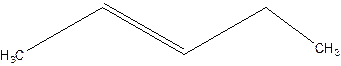
2-pentene.
Explanation of Solution
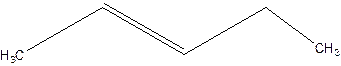
3-Pentene.
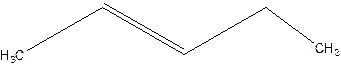
Error- Position of double bond not mentioned correctly.
Correct name-
2-Pentene.
(c)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
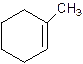
1-Methylcyclo-1-hexene.
Explanation of Solution
2-Methylcyclohexene.
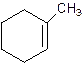
Error- Position of methyl not correct.
Correct name-
1-Methylcyclo-1-hexene.
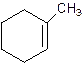
(d)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
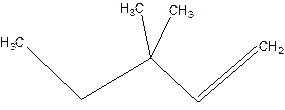
3,3-Dimethyl-1-pentene.
Explanation of Solution
3,3-Dimethylpentene.
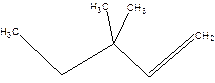
Error-position of double bond not mentioned.
Correct name-
3,3-Dimethyl-1-pentene.
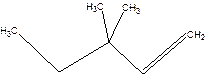
(e)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
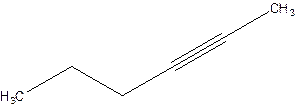
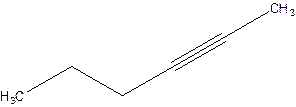
2-Hexyne.
Explanation of Solution
4-Hexyne.
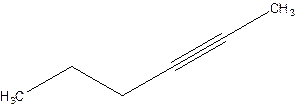
Error- position of triple bond is incorrectly mentioned.
Correct name 2-Hexyne.
(f)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
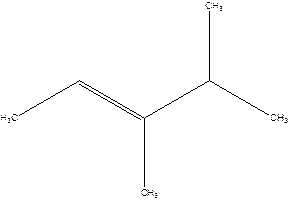
3,4-Dimethyl-2-pentene.
Explanation of Solution
2-Isopropyl-2-butene.
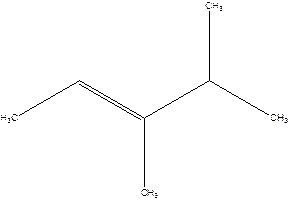
Error- longest chain is not correctly located.
The correct name will be-
3,4-Dimethyl-2-pentene.
Want to see more full solutions like this?
Chapter 12 Solutions
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
- Q8: Draw the resonance structures for the following molecule. Show the curved arrows (how you derive each resonance structure). Circle the major resonance contributor. одarrow_forwardQ9: Explain why compound I is protonated on O while compound II is protonated on N. NH2 DD I II NH2arrow_forwardComplete the following reaction by identifying the principle organic product of the reactionarrow_forward
- Denote the dipole for the indicated bonds in the following molecules. ✓ H3C CH3 B F-CCl3 Br-Cl H3C —Si(CH3)3 CH3 OH HO HO H HO OH vitamin Carrow_forward(a) What is the hybridization of the carbon in the methyl cation (CH3*) and in the methyl anion (CH3)? (b) What is the approximate H-C-H bond angle in the methyl cation and in the methyl anion?arrow_forward10:16 ☑ Vo)) Vo) 4G LTE 76% Complete the following reaction by identifying the principle organic product of the reaction. HO OH ↑ CH2N2 OH ? ○ A. 01 N₂H2C OH ОН B. HO OCH3 OH ○ C. HO OH ŎCH₂N2 ○ D. H3CO OH он Quiz navigation 1 2 3 4 5 11 12 Next page 10 6 7 8 9 10arrow_forward
- Which one of the following statements explain why protecting groups are referred to as “a necessary evil in organic synthesis”? Question 12Select one or more: A. They increase the length and cost of the synthesis B. Every synthesis employs protecting groups C. Protecting group have no role to play in a synthesis D. They minimize the formation of side productsarrow_forwardWhich of the following attributes is a key advantage of the chiral auxiliary approach over the chiral pool approach in asymmetric synthesis? Question 10Select one: A. Chiral auxiliaries are cheaper than chiral pool substrates B. Chiral auxiliary can be recovered and recycled unlike chiral pool substrates. C. The use of chiral auxiliaries provide enantiopure products, while chiral pool reactions are only enantioselective D. The chiral auxiliaries are naturally occurring and do not require synthesisarrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. CH3 CH3 H3C HO: CI:arrow_forward
- Which of the following are TRUE about linear syntheses? Question 7Select one: A. They are easier to execute B. They are the most efficient strategy for all syntheses C. They are generally shorter than convergent syntheses D. They are less versatile compared to convergent synthesesarrow_forwardWhich of the following characteristics is common among chiral pool substrates? Question 4Select one: A. They have good leaving groups B. They are all achiral C. All have a multiplicity of chiral centres D. They have poor leaving groupsarrow_forwardDetermine whether the following reaction is an example of a nucleophilic substitution reaction: H NO2 H+ NO 2 + Molecule A Molecule B Is this a nucleophilic substitution reaction? If this is a nucleophilic substitution reaction, answer the remaining questions in this table. What word or two-word phrase is used to describe the role Molecule A plays in this reaction? What word or two-word phrase is used to describe the role Molecule B plays in this reaction? Use a 6 + symbol to label the electrophilic carbon that is attacked during the substitution. Highlight the leaving group on the appropriate reactant. O Yes ○ No ☐ 0 dx 000 HE ?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

