
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
12th Edition
ISBN: 9781337915984
Author: Bettelheim
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 12, Problem 82P
Interpretation Introduction
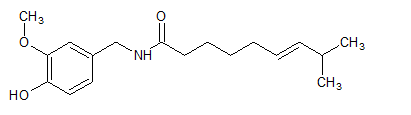
Interpretation: Ways in which capsaicin used in medicine should be explained.
Concept Introduction: The molecular formula of capsaicin is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If we assume a system with an anodic overpotential, the variation of n as a function
of current density:
1. at low fields is linear 2. at higher fields, it follows Tafel's law
Obtain the range of current densities for which the overpotential has the same value
when calculated for 1 and 2 cases (maximum relative difference of 5% compared to
the behavior for higher fields).
To which overpotential range does this correspond?
Data: i = 1.5 mA cm², T = 300°C, B = 0.64, R = 8.314 J K1 mol-1 and F = 96485 C mol-1.
Answer by equation please
Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.
Chapter 12 Solutions
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
Ch. 12.3 - Prob. 12.1QCCh. 12.3 - Prob. 12.2QCCh. 12.3 - Problem 12-3 Write the IUPAC name for each...Ch. 12.3 - Problem 12-4 Draw structural formulas for the...Ch. 12.3 - Problem 12-5 How many stereoisomers are possible...Ch. 12.5 - Prob. 12.6QCCh. 12.5 - Problem 12-7 Propose a two-step mechanism for the...Ch. 12.5 - Prob. 12.8QCCh. 12.5 - Problem 12-9 Propose a three-step reaction...Ch. 12.5 - Prob. 12.10QC
Ch. 12.8 - Prob. 12.11QCCh. 12 - Prob. 1PCh. 12 - Answer true or false. Both ethylene and acetylene...Ch. 12 - 12-13 What is the difference in structure between...Ch. 12 - There are three compounds with the molecular...Ch. 12 - 12-15 Name and draw structural formulas for all...Ch. 12 - Prob. 6PCh. 12 - Draw a structural formula for at least one...Ch. 12 - Each carbon atom in ethane and in ethylene is...Ch. 12 - Prob. 9PCh. 12 - Prob. 10PCh. 12 - Prob. 11PCh. 12 - 12*22 Draw a structural formula for each compound....Ch. 12 - 12-23 Draw a structural formula for each compound....Ch. 12 - Prob. 14PCh. 12 - 12-25 Write the IUPAC name for each unsaturated...Ch. 12 - Explain why each name is incorrect and then write...Ch. 12 - 12-27 Explain why each name is incorrect and then...Ch. 12 - Prob. 18PCh. 12 - 12-29 Which of these alkenes show cis-trans...Ch. 12 - 12-30 Which of these alkenes shows cis-trans...Ch. 12 - 12-31 Cyclodecene exists as both cis and trans...Ch. 12 - Arachidonic acid is a naturally occurring C„o...Ch. 12 - Prob. 23PCh. 12 - If you examine the structural formulas for the...Ch. 12 - 12*35 For each molecule that shows eis-trans...Ch. 12 - Name and draw structural formulas for all...Ch. 12 - /3-Ocimene, a triene found in the fragrance of...Ch. 12 - Answer true or false. Alkenes and alkynes are...Ch. 12 - Prob. 29PCh. 12 - 12-40 Define alkene addition reaction. Write an...Ch. 12 - Prob. 31PCh. 12 - 12-42 Complete these equations.Ch. 12 - Draw structural formulas for all possible...Ch. 12 - Prob. 34PCh. 12 - 12-45 Draw a structural formula for the product of...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - 12-47 Draw a structural formula for an alkene with...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - Prob. 39PCh. 12 - 12-50 Draw the structural formula of an alkene...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Following is the structural formula of...Ch. 12 - Propose an explanation for the following...Ch. 12 - There are nine alkenes with the molecular formula...Ch. 12 - Prob. 46PCh. 12 - 12-57 Hydrocarbon A, Cf,Hs, reacts with 2 moles of...Ch. 12 - 12-58 Show how to convert ethylene to these...Ch. 12 - 12-59 Show how to convert 1-butene to these...Ch. 12 - Prob. 50PCh. 12 - 12-61 (Chemical Connections 12A) What is one...Ch. 12 - Prob. 52PCh. 12 - Prob. 53PCh. 12 - 12-64 (Chemical Connections 120 What is the...Ch. 12 - (Chemical Connections 120 Assume that 1 X IO-12 g...Ch. 12 - Prob. 56PCh. 12 - Prob. 57PCh. 12 - Prob. 58PCh. 12 - Prob. 59PCh. 12 - Prob. 60PCh. 12 - Prob. 61PCh. 12 - Suppose you have unlabeled bottles of benzene and...Ch. 12 - Prob. 63PCh. 12 - Prob. 64PCh. 12 - Answer true or false. (a) Phenols and alcohols...Ch. 12 - Prob. 66PCh. 12 - Prob. 67PCh. 12 - Prob. 68PCh. 12 - Prob. 69PCh. 12 - 12-67 (Chemical Connections 12D ) In which isomer...Ch. 12 - Prob. 71PCh. 12 - Prob. 72PCh. 12 - Prob. 73PCh. 12 - Prob. 74PCh. 12 - Prob. 75PCh. 12 - Prob. 76PCh. 12 - Prob. 77PCh. 12 - Prob. 78PCh. 12 - Prob. 79PCh. 12 - Prob. 80PCh. 12 - Prob. 81PCh. 12 - Prob. 82PCh. 12 - Prob. 83PCh. 12 - Prob. 84PCh. 12 - Prob. 85PCh. 12 - Prob. 86PCh. 12 - Propose a structural formula for the product!s)...Ch. 12 - Prob. 88PCh. 12 - Draw the structural formula of an alkene that...Ch. 12 - 12-77 Show how to convert cyclopentene into these...Ch. 12 - Prob. 91PCh. 12 - Prob. 92PCh. 12 - Prob. 93PCh. 12 - Prob. 94PCh. 12 - Prob. 95PCh. 12 - In omega-3 fatty adds, the last carbon of the last...Ch. 12 - Prob. 97PCh. 12 - Prob. 98P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forward
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY