
Principles of General Organic & Biological Chemistry
2nd Edition
ISBN: 9780077633721
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Question
Chapter 12, Problem 12.72AP
a.
Interpretation Introduction
Interpretation:
The product formed on reduction of below compound has to be drawn.
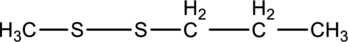
Concept introduction:
Reduction of disulfides:
Thiols are organic compounds which contain a sulfhydryl group (SH group) bonded to atetrahedral carbon. Thiols differ from alcohols, as they are incapable of intermolecular hydrogen bonding. This gives thiols lower boiling points and melting points than alcohols even though having the same size and shape.
In the presence of hydrogen, disulfides are reduced to thiols by gaining hydrogen atoms.
b.
Interpretation Introduction
Interpretation:
The product formed on reduction of below compound has to be drawn
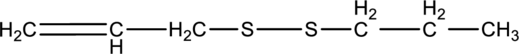
Concept introduction:
Refer to part “a.”.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a tetramer of this alternating copolymer.
H
I
T
H
HH
H
-H
C.
H-
Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above.
H-
H
CIH
H
H
H
HHHH
H
H
0
·H
H–
冊
CH
CHI
HH C-
H-
H
H-
H
H
A.
H
H C
H
H-
-H
HH
H
B.
H-
-H
D.
H
H H
H
•
H
-H
E.
-H
H
H
HICH
T
HHH
F.
H-
Polylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.
Chapter 12 Solutions
Principles of General Organic & Biological Chemistry
Ch. 12.1 - a. Label the hydroxyl groups, thiols, halogens,...Ch. 12.1 - Draw out each compound to clearly show what groups...Ch. 12.2 - Classify each alcohol as 1, 2, or 3.Ch. 12.2 - Classify each hydroxyl group in sorbitol as 1, 2,...Ch. 12.2 - Which compound in each pair has the higher boiling...Ch. 12.2 - Label each compound as water soluble or water...Ch. 12.2 - Give the IUPAC name for each compound.Ch. 12.2 - Give the structure corresponding to each name. a....Ch. 12.3 - Name each ether. a. CH3OCH2CH2CH2CH3 b....Ch. 12.3 - Prob. 12.10P
Ch. 12.3 - Which compound in each pair has the higher boiling...Ch. 12.5 - Prob. 12.12PCh. 12.5 - Prob. 12.13PCh. 12.6 - Prob. 12.14PCh. 12.6 - Prob. 12.15PCh. 12.6 - Give the structure corresponding to each name. a....Ch. 12.7 - Prob. 12.17PCh. 12.8 - Give the IUPAC name for each aldehyde. a....Ch. 12.8 - Prob. 12.19PCh. 12.8 - Give the IUPAC name for each aldehyde depicted in...Ch. 12.8 - Prob. 12.21PCh. 12.8 - Prob. 12.22PCh. 12.8 - Acetone and progesterone are two ketones that...Ch. 12.9 - Prob. 12.24PCh. 12.10 - Prob. 12.25PCh. 12.11 - Prob. 12.26PCh. 12.11 - Prob. 12.27PCh. 12.11 - Prob. 12.28PCh. 12.11 - Prob. 12.29PCh. 12.11 - Prob. 12.30PCh. 12.11 - Prob. 12.31PCh. 12.11 - Prob. 12.32PCh. 12 - Prob. 12.33UKCCh. 12 - Prob. 12.34UKCCh. 12 - Consider the following ball-and-stick model of an...Ch. 12 - Consider the following ball-and-stick model. a....Ch. 12 - Name each compound. a. CH3CH2OCH2CH2CH2CH3Ch. 12 - Name each compound. a. CH3OCH2CH2CH3 b....Ch. 12 - Answer the following questions about alcohol A. a....Ch. 12 - Answer the following questions about alcohol B. a....Ch. 12 - Prob. 12.41UKCCh. 12 - Prob. 12.42UKCCh. 12 - Prob. 12.43UKCCh. 12 - Prob. 12.44UKCCh. 12 - Prob. 12.45APCh. 12 - Prob. 12.46APCh. 12 - Prob. 12.47APCh. 12 - Prob. 12.48APCh. 12 - Prob. 12.49APCh. 12 - Prob. 12.50APCh. 12 - Prob. 12.51APCh. 12 - Prob. 12.52APCh. 12 - Prob. 12.53APCh. 12 - Give the structure corresponding to each name. a....Ch. 12 - Prob. 12.55APCh. 12 - Draw structures for the four constitutional...Ch. 12 - Prob. 12.57APCh. 12 - Rank the following compounds in order of...Ch. 12 - Explain why two four-carbon organic molecules have...Ch. 12 - Explain why the boiling point of CH3CH2CH2CH2OH...Ch. 12 - Which compound in each pair has the higher boiling...Ch. 12 - Which compound in each pair is more water soluble?...Ch. 12 - Prob. 12.63APCh. 12 - Prob. 12.64APCh. 12 - Prob. 12.65APCh. 12 - Prob. 12.66APCh. 12 - Prob. 12.67APCh. 12 - Xylitol is a nontoxic compound as sweet as table...Ch. 12 - Prob. 12.69APCh. 12 - Prob. 12.70APCh. 12 - Prob. 12.71APCh. 12 - Prob. 12.72APCh. 12 - Prob. 12.73APCh. 12 - Prob. 12.74APCh. 12 - Prob. 12.75APCh. 12 - Prob. 12.76APCh. 12 - Prob. 12.77APCh. 12 - Draw the structure corresponding to each name. a....Ch. 12 - Prob. 12.79APCh. 12 - Prob. 12.80APCh. 12 - What product is formed when each compound is...Ch. 12 - Prob. 12.82APCh. 12 - Prob. 12.83APCh. 12 - Prob. 12.84APCh. 12 - Prob. 12.85APCh. 12 - Prob. 12.86APCh. 12 - Prob. 12.87APCh. 12 - Label each of the following objects as chiral or...Ch. 12 - Prob. 12.89APCh. 12 - Prob. 12.90APCh. 12 - Prob. 12.91APCh. 12 - Prob. 12.92APCh. 12 - Prob. 12.93APCh. 12 - Prob. 12.94APCh. 12 - Prob. 12.95APCh. 12 - Prob. 12.96APCh. 12 - Prob. 12.97APCh. 12 - How are the compounds in each pair related? Are...Ch. 12 - Prob. 12.99APCh. 12 - Prob. 12.100APCh. 12 - Prob. 12.101APCh. 12 - Prob. 12.102APCh. 12 - Prob. 12.103APCh. 12 - Lactic acid [CH3CH(OH)CO2H] gives sour milk its...Ch. 12 - Prob. 12.105APCh. 12 - Prob. 12.106APCh. 12 - Prob. 12.107CPCh. 12 - Prob. 12.108CPCh. 12 - Prob. 12.109BTCCh. 12 - Prob. 12.110BTC
Knowledge Booster
Similar questions
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
- Identify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning