
Concept explainers
(a)
Interpretation:
The mass spectrum fragmentation of ethyl bromide at
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of mass of compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. This technique did not interact with
Answer to Problem 12.42AP
The peak at
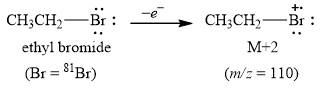
Explanation of Solution
The molecule ethyl bromide contains carbon, hydrogen, bromide atoms. In this molecule carbon and hydrogen mainly exist in one isotope form

Figure 1
The peak at
The mass spectrum fragmentation of ethyl bromide at
(b)
Interpretation:
The mass spectrum fragmentation of ethyl bromide at
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of mass of compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. This technique did not interact with electromagnetic radiation. Two peaks are used to identify the compound, first, the molecular ion peak which is the mass of the compound and second, the base peak which is the most abundant element peak. It may be same or different.
Answer to Problem 12.42AP
The peak at
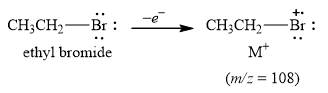
Explanation of Solution
The mass of the compound,

Figure 2
The mass spectrum fragmentation of ethyl bromide at
(c)
Interpretation:
The mass spectrum fragmentation of ethyl bromide at
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of mass of compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. This technique did not interact with electromagnetic radiation. Two peaks are used to identify the compound, first, the molecular ion peak which is the mass of the compound and second, the base peak which is the most abundant element peak. It may be same or different.
Answer to Problem 12.42AP
The peak at
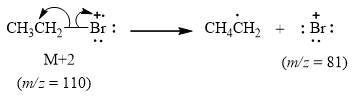
Explanation of Solution
When the ethyl bromide

Figure 3
The mass spectrum fragmentation of ethyl bromide at
(d)
Interpretation:
The mass spectrum fragmentation of ethyl bromide at
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of mass of compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. This technique did not interact with electromagnetic radiation. Two peaks are used to identify the compound, first, the molecular ion peak which is the mass of the compound and second, the base peak which is the most abundant element peak. It may be same or different.
Answer to Problem 12.42AP
The peak at
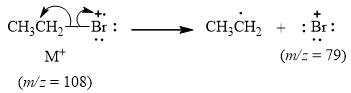
Explanation of Solution
When the ethyl bromide

Figure 4
The mass spectrum fragmentation of ethyl bromide at
(e)
Interpretation:
The mass spectrum fragmentation of ethyl bromide at
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of mass of compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. This technique did not interact with electromagnetic radiation. Two peaks are used to identify the compound, first, the molecular ion peak which is the mass of the compound and second, the base peak which is the most abundant element peak. It may be same or different.
Answer to Problem 12.42AP
The peak at
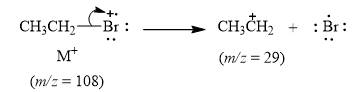
Explanation of Solution
When the ethyl bromide breaks into fragment, it releases ethyl cation

Figure 5
The mass spectrum fragmentation of ethyl bromide at
(f)
Interpretation:
The mass spectrum fragmentation of ethyl bromide at
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of mass of compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. This technique did not interact with electromagnetic radiation. Two peaks are used to identify the compound, first, the molecular ion peak which is the mass of the compound and second, the base peak which is the most abundant element peak. It may be same or different.
Answer to Problem 12.42AP
The peak at
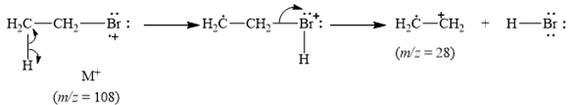
Explanation of Solution
When the ethyl bromide breaks into fragment, it releases ethyl radical cation

Figure 6
The mass spectrum fragmentation of ethyl bromide at
(g)
Interpretation:
The mass spectrum fragmentation of ethyl bromide at
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of mass of compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. This technique did not interact with electromagnetic radiation. Two peaks are used to identify the compound, first, the molecular ion peak which is the mass of the compound and second, the base peak which is the most abundant element peak. It may be same or different.
Answer to Problem 12.42AP
The peak at
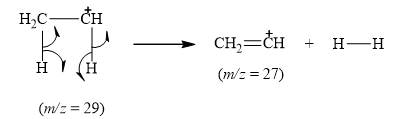
Explanation of Solution
When the ethyl bromide breaks into fragment, it releases ethene cation

Figure 7
The mass spectrum fragmentation of ethyl bromide at
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry
- Why does the anhydride react with the OH on the benzene rather than the OH on the carboxy group?arrow_forwardAnswer the followings: 1- What is the IP for a amino acid? Give example. 2- What are the types of amino acids? 3- What are the structures of protein? 4- The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N- terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin? 5. MATCH a term from the list below to each definition. Place the letter of the term in the blank to the left of the definition. a. Ligases b. Fibrous proteins c. Conjugated protein d. Hydrolases a. b. C. e. Simple protein f. Globular proteins g. Lyases h. Transferases Proteins that are tough and insoluble in water. Enzymes that catalyze the breaking away of a small molecule such as from a substrate. Enzymes that catalyze the bonding together of two substrates.arrow_forwardAnswer the followings (Four): 1-What is the difference(s) between FOUR: a. Glyceride and phosphoglyceride. b. Wax and fat. c. Soap and fatty acid. d. HDL and LDL cholesterol e. Phospho lipids and sphingosine. 2-What are the types of lipids? 3-What are the main lipid components of membrane structures? 4-How could lipids play important rules as signaling molecules and building units? 5. The Structure variety of Lipids makes them to play significant rules in our body. Conclude briefly on this statement.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





