
(a)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(b)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(c)
Interpretation:
Whether the cyclohexane ring “breathing” or simultaneous stretching of the all
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The simultaneous stretch of all
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The simultaneous stretch of all
Therefore, simultaneous stretch of all
The simultaneous stretch of all
(d)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(e)
Interpretation:
Whether the symmetrical stretching of the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The symmetric stretch of
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The symmetric stretch of
Therefore, symmetric stretch of
The symmetric stretch of
(f)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The stretch of
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The stretch of
Therefore, stretch of
The stretch of
(g)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The structure of
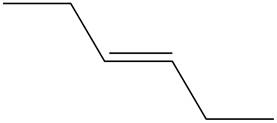
Figure 1
The
Therefore,
The
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry
- 31 Indicate the symbol, mass number and the atomic number of the missing product in each of the following nuclear reactions. a) 13/3 N 41 b) 11 Ca 20 c) 90 38 Sr → 133 C + ? + - 6 0 e →? 90 Y + ? 39 11 d) 22 Na → ? + + 1 B +1 β Toarrow_forwardPlease drawarrow_forward9. compore the Following two Venctions IN termy Of Ronction Rate and explan in detail the reasoning that led to your conclusion +He p₁₂ 11- ㅐ 15 .. +He H #H H / H b. Compare the Following too reactions 14 terms of reaction Rate and explain in detail the reasoning that led to your conclusion Н d-C- tłu Na +2446 е -ll +2n "Harrow_forward
- a. •Write all of the possible products For the Following ronction А ----- H - H H + H₂0 H+ Н b. in Rite the complete reaction Mechaniszn For the Formation of each product. ·C. Suggest what Reaction conditions could Result in each product being the major Product of the veaction:arrow_forwarda. Write the product For each of the Following reactions H 6-836-6 레 +H₂ N A H A-C-C=C-C-CH + 2 Na +2 NH3 - H H b. Write the reaction Mechanism For. reaction eacharrow_forwardhelp draw the moleculearrow_forward
- How to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forward
- a. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forwardhelp draw any straightchain moleculearrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
