
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.31P
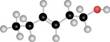
Devise a synthesis of the following compound from acetylene and organic compounds containing two carbons or fewer. You may use any other required reagents.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
NMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at
4.1 ppm? Select the single best answer.
The
H
O
HỌC—C—0—CH, CH,
2
A
ethyl acetate
H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm
Check
OA
B
OC
ch
B
C
Save For Later
Submit Ass
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
How many signals do you expect in the H NMR spectrum for this molecule?
Br Br
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red
Note for advanced students: In this question, any multiplet is counted as one signal.
1
Number of signals in the 'H NMR spectrum.
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
Check
For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute
to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
O
✓
No additional Hs to color in top
molecule
ง
No additional Hs to color in bottom…
in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstant
Chapter 12 Solutions
Organic Chemistry-Package(Custom)
Ch. 12 - Prob. 12.1PCh. 12 - Prob. 12.2PCh. 12 - Prob. 12.3PCh. 12 - Prob. 12.4PCh. 12 - Prob. 12.5PCh. 12 - Given that syn addition of H2 occurs from both...Ch. 12 - Compound Molecular formula before...Ch. 12 - Draw the products formed when triacylglycerol A is...Ch. 12 - Prob. 12.9PCh. 12 - Prob. 12.10P
Ch. 12 - Problem 12.11 (a) Draw the structure of a compound...Ch. 12 - Prob. 12.12PCh. 12 - Prob. 12.13PCh. 12 - Prob. 12.14PCh. 12 - Prob. 12.15PCh. 12 - Prob. 12.16PCh. 12 - Prob. 12.17PCh. 12 - Problem 12.18 Draw the products formed when both...Ch. 12 - Prob. 12.19PCh. 12 - Prob. 12.20PCh. 12 - Prob. 12.21PCh. 12 - Prob. 12.22PCh. 12 - Prob. 12.23PCh. 12 - Problem 12.24 Draw the organic products in each of...Ch. 12 - Prob. 12.25PCh. 12 - Prob. 12.26PCh. 12 - Problem 12.27 Draw the products of each Sharpless...Ch. 12 - Prob. 12.28PCh. 12 - 12.29 Draw the products formed when A is treated...Ch. 12 - Prob. 12.30PCh. 12 - 12.31 Devise a synthesis of the following compound...Ch. 12 - Label each reaction as oxidation, reduction, or...Ch. 12 - Prob. 12.33PCh. 12 - Prob. 12.34PCh. 12 - Prob. 12.35PCh. 12 - Prob. 12.36PCh. 12 - 12.37 Stearidonic acid (C18H28O2) is an...Ch. 12 - Draw the organic products formed when cyclopentene...Ch. 12 - Prob. 12.39PCh. 12 - Draw the organic products formed when allylic...Ch. 12 - Draw the organic products formed in each reaction...Ch. 12 - Draw the organic products formed in each reaction....Ch. 12 - Prob. 12.43PCh. 12 - Prob. 12.44PCh. 12 - Prob. 12.45PCh. 12 - What alkene is needed to synthesize each 1,2-diol...Ch. 12 - Prob. 12.47PCh. 12 - Draw the products formed after Steps 1 and 2 in...Ch. 12 - Prob. 12.49PCh. 12 - Prob. 12.50PCh. 12 - Prob. 12.51PCh. 12 - What alkyne gives each set of products after...Ch. 12 - Prob. 12.53PCh. 12 - Prob. 12.54PCh. 12 - Prob. 12.55PCh. 12 - 12.54 An unknown compound A of molecular formula ...Ch. 12 - 12.55 DHA is a fatty acid derived from fish oil...Ch. 12 - Prob. 12.58PCh. 12 - Prob. 12.59PCh. 12 - 12.58 Epoxidation of the following allylic alcohol...Ch. 12 - What allylic alcohol and DET isomer are needed to...Ch. 12 - Devise a synthesis of each hydrocarbon from...Ch. 12 - Prob. 12.63PCh. 12 - 12.62 It is sometimes necessary to isomerize a cis...Ch. 12 - Prob. 12.65PCh. 12 - Prob. 12.66PCh. 12 - Prob. 12.67PCh. 12 - Prob. 12.68PCh. 12 - Devise a synthesis of each compound from the...Ch. 12 - Devise a synthesis of each compound from acetylene...Ch. 12 - Prob. 12.71PCh. 12 - Prob. 12.72PCh. 12 - Prob. 12.73PCh. 12 - Prob. 12.74PCh. 12 - Prob. 12.75PCh. 12 - Prob. 12.76PCh. 12 - 12.72 Draw a stepwise mechanism for the following...Ch. 12 - Prob. 12.78PCh. 12 - Prob. 12.79PCh. 12 - Prob. 12.80PCh. 12 - 12.75 Sharpless epoxidation of allylic alcohol X...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License