![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)
11th Edition
ISBN: 9781305106734
Author: Frederick A. Bettelheim; William H. Brown; Mary K. Campbell; Shawn O. Farrell; Omar Torres
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.25P
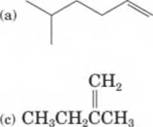
12-25 Write the IUPAC name for each
(d)c=ch2
ch3ch,ch2
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
+
C8H16O2 (Fatty acid) +
11 02 → 8 CO2
a. Which of the above are the reactants?
b. Which of the above are the products?
H2o CO₂
c. Which reactant is the electron donor? Futty acid
d. Which reactant is the electron acceptor?
e. Which of the product is now reduced?
f. Which of the products is now oxidized?
02
#20
102
8 H₂O
g. Where was the carbon initially in this chemical reaction and where is it now that it is
finished?
2
h. Where were the electrons initially in this chemical reaction and where is it now that it is
finished?
→
Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP
a. Which of the above are the reactants?
b. Which of the above are the products?
c. Which reactant is the electron donor?
d. Which reactants are the electron acceptors?
e. Which of the products are now reduced?
f. Which product is now oxidized?
g. Which process was used to produce the ATP?
h. Where was the energy initially in this chemical reaction and where is it now that it is
finished?
i. Where was the carbon initially in this chemical reaction and where is it now that it is
finished?
j. Where were the electrons initially in this chemical reaction and where is it now that it is
finished?
Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic
aromatic substitution.
OCH 3
(Choose one)
OH
(Choose one)
Br
(Choose one)
Explanation
Check
NO2
(Choose one)
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A
Chapter 12 Solutions
OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)
Ch. 12.3 - Prob. 12.1PCh. 12.3 - Prob. 12.2PCh. 12.3 - Problem 12-3 Write the IUPAC name for each...Ch. 12.3 - Problem 12-4 Draw structural formulas for the...Ch. 12.3 - Problem 12-5 How many stereoisomers are possible...Ch. 12.5 - Prob. 12.6PCh. 12.5 - Problem 12-7 Propose a two-step mechanism for the...Ch. 12.5 - Prob. 12.8PCh. 12.5 - Problem 12-9 Propose a three-step reaction...Ch. 12.5 - Prob. 12.10P
Ch. 12 - Prob. 12.11PCh. 12 - Answer true or false. Both ethylene and acetylene...Ch. 12 - 12-13 What is the difference in structure between...Ch. 12 - There are three compounds with the molecular...Ch. 12 - 12-15 Name and draw structural formulas for all...Ch. 12 - Prob. 12.16PCh. 12 - Draw a structural formula for at least one...Ch. 12 - Each carbon atom in ethane and in ethylene is...Ch. 12 - Prob. 12.19PCh. 12 - Prob. 12.20PCh. 12 - Prob. 12.21PCh. 12 - 12*22 Draw a structural formula for each compound....Ch. 12 - 12-23 Draw a structural formula for each compound....Ch. 12 - Prob. 12.24PCh. 12 - 12-25 Write the IUPAC name for each unsaturated...Ch. 12 - Explain why each name is incorrect and then write...Ch. 12 - 12-27 Explain why each name is incorrect and then...Ch. 12 - Prob. 12.28PCh. 12 - 12-29 Which of these alkenes show cis-trans...Ch. 12 - 12-30 Which of these alkenes shows cis-trans...Ch. 12 - 12-31 Cyclodecene exists as both cis and trans...Ch. 12 - Arachidonic acid is a naturally occurring C„o...Ch. 12 - Prob. 12.33PCh. 12 - If you examine the structural formulas for the...Ch. 12 - 12*35 For each molecule that shows eis-trans...Ch. 12 - Name and draw structural formulas for all...Ch. 12 - /3-Ocimene, a triene found in the fragrance of...Ch. 12 - Answer true or false. Alkenes and alkynes are...Ch. 12 - Prob. 12.39PCh. 12 - 12-40 Define alkene addition reaction. Write an...Ch. 12 - Prob. 12.41PCh. 12 - 12-42 Complete these equations.Ch. 12 - Draw structural formulas for all possible...Ch. 12 - Prob. 12.44PCh. 12 - 12-45 Draw a structural formula for the product of...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - 12-47 Draw a structural formula for an alkene with...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - Prob. 12.49PCh. 12 - 12-50 Draw the structural formula of an alkene...Ch. 12 - Prob. 12.51PCh. 12 - Prob. 12.52PCh. 12 - Following is the structural formula of...Ch. 12 - Propose an explanation for the following...Ch. 12 - There are nine alkenes with the molecular formula...Ch. 12 - Prob. 12.56PCh. 12 - 12-57 Hydrocarbon A, Cf,Hs, reacts with 2 moles of...Ch. 12 - 12-58 Show how to convert ethylene to these...Ch. 12 - 12-59 Show how to convert 1-butene to these...Ch. 12 - Prob. 12.60PCh. 12 - 12-61 (Chemical Connections 12A) What is one...Ch. 12 - Prob. 12.62PCh. 12 - Prob. 12.63PCh. 12 - 12-64 (Chemical Connections 120 What is the...Ch. 12 - (Chemical Connections 120 Assume that 1 X IO-12 g...Ch. 12 - Prob. 12.66PCh. 12 - 12-67 (Chemical Connections 12D ) In which isomer...Ch. 12 - Prob. 12.68PCh. 12 - Prob. 12.69PCh. 12 - Prob. 12.70PCh. 12 - Prob. 12.71PCh. 12 - Prob. 12.72PCh. 12 - Prob. 12.73PCh. 12 - Propose a structural formula for the product!s)...Ch. 12 - Prob. 12.75PCh. 12 - Draw the structural formula of an alkene that...Ch. 12 - 12-77 Show how to convert cyclopentene into these...Ch. 12 - Prob. 12.78PCh. 12 - Prob. 12.79PCh. 12 - In omega-3 fatty adds, the last carbon of the last...Ch. 12 - Prob. 12.81P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardIdentifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Assign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forwarddescrive the energy levels of an atom and howan electron moces between themarrow_forwardRank each set of substituents using the Cahn-Ingold-Perlog sequence rules (priority) by numbering the highest priority substituent 1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY