![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
Concept explainers
12-42 Complete these equations.
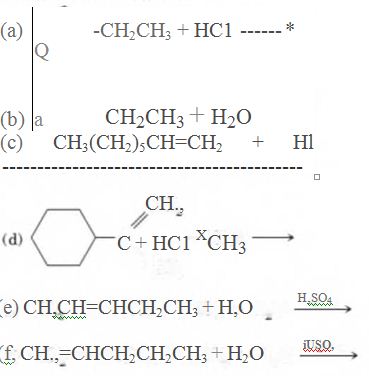
(a)
Interpretation:
Complete the below equation:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 1](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_1.png)
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and Cl- ions. This reaction is carried out in the presence of acid therefore it is called acid catalyzed hydration of alkene.
Answer to Problem 12.42P
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 2](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_2.png)
Explanation of Solution
As per the given equation, the reactant is a cyclic alkene to which HCl is added. And the characteristics reactions given by the alkenes are addition reactions in which the addition takes place at the double bond. The addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is Cl-. On numbering the carbon atoms, the structure is.
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 3](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_3.png)
Now as per the rule, the hydrogen in the above equation attaches to carbon 2 as carbon 2 of the double bond has one hydrogen attached to it. The Cl- will attached to carbon 1 as it has no carbon attached to it. Thus, the complete reaction is:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 4](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_4.png)
(b)
Interpretation:
Complete the below equation:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 5](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_5.png)
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and OH- ions in the presence of H2 SO4. This reaction is carried out in the presence of acid therefore it is called acid catalyzed hydration of alkene.
Answer to Problem 12.42P
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 6](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_6.png)
Explanation of Solution
As per the given equation, the reactant is a cyclic alkene to which H2 O is added. And the characteristics reactions given by the alkenes are addition reactions in which the addition takes place at the double bond. The addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is OH-. On numbering the carbon atoms, the structure is.
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 7](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_7.png)
Now as per the rule, the hydrogen in the above equation attaches to carbon 2 as carbon 2 of the double bond has one hydrogen attached to it. The OH- will attached to carbon 1 as it has no carbon attached to it. Thus, the complete reaction is:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 8](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_8.png)
(c)
Interpretation:
Complete the below equation:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 9](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_9.png)
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and I- ions.
Answer to Problem 12.42P
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 10](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_10.png)
Explanation of Solution
As per the above equation, the reactant is an alkene to which HI is added. We also know that the characteristics reactions given by alkenes are addition reactions in which the addition takes place at the double bond. This addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is I-. On numbering the double bonded carbons of the alkene, the structure is.
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 11](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_11.png)
Now as per the rule, the hydrogen in the above equation attaches to carbon 1 as carbon 1 of the double bond has two hydrogen attached to it. The I- will attached to carbon 2 as it has only one carbon attached to it. Thus, the complete reaction is:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 12](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_12.png)
(d)
Interpretation:
Complete the below equation:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 13](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_13.png)
Concept introduction:
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it.
Answer to Problem 12.42P
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 14](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_14.png)
Explanation of Solution
As per the above equation, the reactant is an alkene to which HCl is added. We also know that the characteristics reactions given by alkenes are addition reactions in which the addition takes place at the double bond. This addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is Cl-. On numbering the double bonded carbons of the alkene, the structure is.
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 15](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_15.png)
Now as per the rule, the hydrogen in the above equation attaches to carbon 1 as carbon 1 of the double bond has two hydrogen attached to it. The Cl- will attached to carbon 2 as it has only no carbon attached to it. Thus, the complete reaction is:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 16](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_16.png)
(e)
Interpretation:
Complete the below equation:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 17](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_17.png)
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and OH- ions in the presence of H2 SO4. This reaction is carried out in the presence of acid therefore it is called acid catalyzed hydration of alkene.
Answer to Problem 12.42P
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 18](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_18.png)
Explanation of Solution
As per the above equation, the reactant is an alkene to which H2 O is added. We also know that the characteristics reactions given by alkenes are addition reactions in which the addition takes place at the double bond. This addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is OH-. On numbering the alkene, the structure is.
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 19](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_19.png)
Now the below product will form where hydrogen will attach to the 2nd carbon to form more suitable product:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 20](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_20.png)
(f)
Interpretation:
Complete the below equation:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 21](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_21.png)
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and OH- ions in the presence of H2 SO4. This reaction is carried out in the presence of acid therefore it is called acid catalyzed hydration of alkene.
Answer to Problem 12.42P
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 22](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_22.png)
Explanation of Solution
As per the above equation, the reactant is an alkene to which H2 O is added. We also know that the characteristics reactions given by alkenes are addition reactions in which the addition takes place at the double bond. This addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is OH-. On numbering the alkene, the structure is.
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 23](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_23.png)
Now as per the rule, the hydrogen in the above equation attaches to carbon 1 as carbon 1 of the double bond has two hydrogen attached to it. The OH- will attached to carbon 2 as it has only one carbon attached to it. Thus, the complete reaction is:
![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months), Chapter 12, Problem 12.42P , additional homework tip 24](https://content.bartleby.com/tbms-images/9781285869759/Chapter-12/images/html_69759-12-12.42p_24.png)
Want to see more full solutions like this?
Chapter 12 Solutions
OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
- Identify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co



