![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)
11th Edition
ISBN: 9781305106734
Author: Frederick A. Bettelheim; William H. Brown; Mary K. Campbell; Shawn O. Farrell; Omar Torres
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.43P
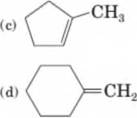
Draw structural formulas for all possible carbocations formed by the reaction of each
ch3
I
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.
Synthesis of Dibenzalacetone
[References]
Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below.
Question 1
1 pt
Question 2
1 pt
Question 3
1 pt
H
Question 4
1 pt
Question 5
1 pt
Question 6
1 pt
Question 7
1pt
Question 8
1 pt
Progress:
7/8 items
Que Feb 24 at
You do not have to consider stereochemistry.
. Draw the enolate ion in its carbanion form.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
⚫ Separate multiple reactants using the + sign from the drop-down menu.
?
4
Shown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)
Chapter 12 Solutions
OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)
Ch. 12.3 - Prob. 12.1PCh. 12.3 - Prob. 12.2PCh. 12.3 - Problem 12-3 Write the IUPAC name for each...Ch. 12.3 - Problem 12-4 Draw structural formulas for the...Ch. 12.3 - Problem 12-5 How many stereoisomers are possible...Ch. 12.5 - Prob. 12.6PCh. 12.5 - Problem 12-7 Propose a two-step mechanism for the...Ch. 12.5 - Prob. 12.8PCh. 12.5 - Problem 12-9 Propose a three-step reaction...Ch. 12.5 - Prob. 12.10P
Ch. 12 - Prob. 12.11PCh. 12 - Answer true or false. Both ethylene and acetylene...Ch. 12 - 12-13 What is the difference in structure between...Ch. 12 - There are three compounds with the molecular...Ch. 12 - 12-15 Name and draw structural formulas for all...Ch. 12 - Prob. 12.16PCh. 12 - Draw a structural formula for at least one...Ch. 12 - Each carbon atom in ethane and in ethylene is...Ch. 12 - Prob. 12.19PCh. 12 - Prob. 12.20PCh. 12 - Prob. 12.21PCh. 12 - 12*22 Draw a structural formula for each compound....Ch. 12 - 12-23 Draw a structural formula for each compound....Ch. 12 - Prob. 12.24PCh. 12 - 12-25 Write the IUPAC name for each unsaturated...Ch. 12 - Explain why each name is incorrect and then write...Ch. 12 - 12-27 Explain why each name is incorrect and then...Ch. 12 - Prob. 12.28PCh. 12 - 12-29 Which of these alkenes show cis-trans...Ch. 12 - 12-30 Which of these alkenes shows cis-trans...Ch. 12 - 12-31 Cyclodecene exists as both cis and trans...Ch. 12 - Arachidonic acid is a naturally occurring C„o...Ch. 12 - Prob. 12.33PCh. 12 - If you examine the structural formulas for the...Ch. 12 - 12*35 For each molecule that shows eis-trans...Ch. 12 - Name and draw structural formulas for all...Ch. 12 - /3-Ocimene, a triene found in the fragrance of...Ch. 12 - Answer true or false. Alkenes and alkynes are...Ch. 12 - Prob. 12.39PCh. 12 - 12-40 Define alkene addition reaction. Write an...Ch. 12 - Prob. 12.41PCh. 12 - 12-42 Complete these equations.Ch. 12 - Draw structural formulas for all possible...Ch. 12 - Prob. 12.44PCh. 12 - 12-45 Draw a structural formula for the product of...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - 12-47 Draw a structural formula for an alkene with...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - Prob. 12.49PCh. 12 - 12-50 Draw the structural formula of an alkene...Ch. 12 - Prob. 12.51PCh. 12 - Prob. 12.52PCh. 12 - Following is the structural formula of...Ch. 12 - Propose an explanation for the following...Ch. 12 - There are nine alkenes with the molecular formula...Ch. 12 - Prob. 12.56PCh. 12 - 12-57 Hydrocarbon A, Cf,Hs, reacts with 2 moles of...Ch. 12 - 12-58 Show how to convert ethylene to these...Ch. 12 - 12-59 Show how to convert 1-butene to these...Ch. 12 - Prob. 12.60PCh. 12 - 12-61 (Chemical Connections 12A) What is one...Ch. 12 - Prob. 12.62PCh. 12 - Prob. 12.63PCh. 12 - 12-64 (Chemical Connections 120 What is the...Ch. 12 - (Chemical Connections 120 Assume that 1 X IO-12 g...Ch. 12 - Prob. 12.66PCh. 12 - 12-67 (Chemical Connections 12D ) In which isomer...Ch. 12 - Prob. 12.68PCh. 12 - Prob. 12.69PCh. 12 - Prob. 12.70PCh. 12 - Prob. 12.71PCh. 12 - Prob. 12.72PCh. 12 - Prob. 12.73PCh. 12 - Propose a structural formula for the product!s)...Ch. 12 - Prob. 12.75PCh. 12 - Draw the structural formula of an alkene that...Ch. 12 - 12-77 Show how to convert cyclopentene into these...Ch. 12 - Prob. 12.78PCh. 12 - Prob. 12.79PCh. 12 - In omega-3 fatty adds, the last carbon of the last...Ch. 12 - Prob. 12.81P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forwardDraw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY