
Concept explainers
(a)
Interpretation:
The given statement is true or false should be determined.
The IUPAC name of an alkene is derived from the name of the longest carbon chain that contains the carbon-carbon double bond.
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism. If same groups are present on the same side, then the isomer is called as cis-isomer and the same groups are present on different side the isomer is called as trans-isomer.
Answer to Problem 12.21P
The given statement is true.
Explanation of Solution
As per general rules of
(b)
Interpretation:
The given statement is true or false should be determined.
The IUPAC name of
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side, then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is false.
Explanation of Solution
IUPAC name of
Therefore, the given statement is false.
(c)
Interpretation:
The given statement is true or false should be determined.
2-Methyl-2-butene shows cis-trans isomerism.
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side, then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is false.
Explanation of Solution
2-Methyl-2-butene contains two methyl groups present on carbon atom of carbon-carbon double bond other carbon contains also methyl group.
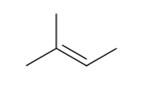
To get cis and trans isomers, two different groups should be present on the carbon atoms of the carbons with double bond.
Thus, the statement is false.
(d)
Interpretation:
The given statement is true or false should be determined.
1,2-dimethylcyclohexene shows cis-trans isomerism.
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is false.
Explanation of Solution
Following are the structures of the isomers of 1,2-dimethylcyclohexane. Form this, we can see that all four atoms of carbon-carbon double bond are lying in same plane.
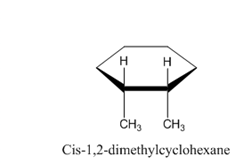
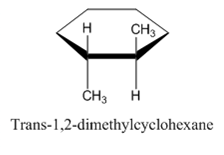
Therefore, the given statement is false.
(e)
Interpretation:
The given statement is true or false should be determined.
The IUPAC name of
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is true.
Explanation of Solution
The IUPAC name of
(f)
Interpretation:
The given statement is true or false should be determined.
1,3-Butadiene has two carbon-carbon double bonds and 22 =4 stereoisomers are possible for it.
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side, then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is false.
Explanation of Solution
Following is the structure of 1,3-Butadiene. There are no stereocenters are present in the structure of 1,3-Butadiene. There should be presence of different groups for isomerism.
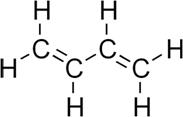
Therefore, the given statement is false.
Want to see more full solutions like this?
Chapter 12 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
- Please correct answer and don't used hand raiting and don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardThe vibrational contribution isa) temperature independent for internal energy and heat capacityb) temperature dependent for internal energy and heat capacityc) temperature independent for heat capacityd) temperature independent for internal energyarrow_forward
- Quantum mechanics. Explain the basis of approximating the summation to an integral in translational motion.arrow_forwardQuantum mechanics. In translational motion, the summation is replaced by an integral when evaluating the partition function. This is correct becausea) the spacing of the translational energy levels is very small compared to the product kTb) the spacing of the translational energy levels is comparable to the product kTc) the spacing of the translational energy levels is very large compared to the product kTarrow_forwardDon't used Ai solutionarrow_forward
- Please correct answer and don't used hand raiting don't used Ai solutionarrow_forwardIf the viscosity of hydrogen gas (at 0oC and 1 atm) is 8.83x10-5 P. If we assume that the molecular sizes are equal, calculate the viscosity of a gas composed of deuterium.arrow_forwardIf the viscosity of hydrogen gas (at 0oC and 1 atm) is 8.83x10-5 P. If we assume that the molecular sizes are equal, calculate the viscosity of a gas composed of deuterium.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




