
LCPO CHEMISTRY W/MODIFIED MASTERING
8th Edition
ISBN: 9780135214756
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 12, Problem 12.103SP
Interpretation Introduction
(a)
Interpretation:
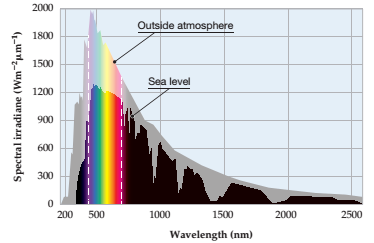
The color and approximate wavelength of maximum solar intensity at the Earth’s surface should be determined.
Concept introduction:
Solar irradiance spectrum above atmosphere and at surface.
Interpretation Introduction
(b)
Interpretation:
The semiconductor that absorb at a wavelength matched with maximum solar intensity should be determined.
Concept introduction:
The band gap energy can be represented as follows:
Here,
E = band gap energy
h = Planck’s constant
c =
λ = wavelength
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the structure of the major organic product(s) of the reaction.
H3C
1. DIBAH, toluene
-CH3
+
2. H3O
DIBAH = diisobutylaluminum hydride,
[(CH3)2CHCH2]2AIH
Which of the following is not an intermediate of the reaction below? Why is the correct answer C? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.
Which of the following is the product of the reaction between acetone, CH3COCH3 and methyl amine, CH3NH2? Why is the correct answer A? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.
Chapter 12 Solutions
LCPO CHEMISTRY W/MODIFIED MASTERING
Ch. 12 - Calcium metal crystallizes in a cubic...Ch. 12 - Polonium metal crystallizes in a simple cubic...Ch. 12 - Polonium metal crystallizes in a simple cubic...Ch. 12 - The density of a sample of metal "as measured to...Ch. 12 - Zinc sulfide crystallizes in the following cubic...Ch. 12 - Prob. 12.6ACh. 12 - Prob. 12.7PCh. 12 - Prob. 12.8ACh. 12 - Prob. 12.9PCh. 12 - Prob. 12.10A
Ch. 12 - Prob. 12.11PCh. 12 - Prob. 12.12ACh. 12 - Prob. 12.13PCh. 12 - Prob. 12.14PCh. 12 - Prob. 12.15PCh. 12 - Prob. 12.16PCh. 12 - Prob. 12.17PCh. 12 - Identify each of the following kinds of packingCh. 12 - Prob. 12.19CPCh. 12 - Titanium oxide crystallizes in the following cubic...Ch. 12 - Prob. 12.21CPCh. 12 - Prob. 12.22CPCh. 12 - Prob. 12.23CPCh. 12 - Prob. 12.24CPCh. 12 - Prob. 12.25CPCh. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - Prob. 12.29SPCh. 12 - Prob. 12.30SPCh. 12 - Prob. 12.31SPCh. 12 - Diffraction of X rays with =154.2 pm at an angle...Ch. 12 - Diffraction of X rays with =154.2 pm at an angle...Ch. 12 - Which of the four kinds of packing used by metals...Ch. 12 - What is a unit cell? How many atoms are in one...Ch. 12 - Copper crystallizes in a face-centered cubic unit...Ch. 12 - Lead crystallizes in a cubic unit cell with anedge...Ch. 12 - Prob. 12.38SPCh. 12 - Tungsten crystallizes in a body-centered cubic...Ch. 12 - Prob. 12.40SPCh. 12 - Prob. 12.41SPCh. 12 - Titanium metal has a density of and an atomic...Ch. 12 - Calcium metal has a density of 1.55 g/cm3 and...Ch. 12 - The atomic radius of Pb is 175 pm, and the density...Ch. 12 - The density of a sample of metal was measured to...Ch. 12 - If a protein can be induced to crystallize, its...Ch. 12 - The molecular structure of a scorpion toxin, a...Ch. 12 - Iron crystallizes in a body-centered cubic unit...Ch. 12 - Silver metal crystallizes in a face-centered cubic...Ch. 12 - Sodium hydride, NaH, crystallizes in a...Ch. 12 - Cesium chloride crystallizers in a cubic unit cell...Ch. 12 - If the edge length of an NaH unit cell is 488 pm,...Ch. 12 - The edge length of a CsCI unit cell (Problem...Ch. 12 - Silicon carbide, SiC, is a covalent network solid...Ch. 12 - Prob. 12.55SPCh. 12 - Prob. 12.56SPCh. 12 - Prob. 12.57SPCh. 12 - Prob. 12.58SPCh. 12 - Prob. 12.59SPCh. 12 - Prob. 12.60SPCh. 12 - Prob. 12.61SPCh. 12 - Prob. 12.62SPCh. 12 - Prob. 12.63SPCh. 12 - Prob. 12.64SPCh. 12 - Prob. 12.65SPCh. 12 - Prob. 12.66SPCh. 12 - Prob. 12.67SPCh. 12 - Prob. 12.68SPCh. 12 - Prob. 12.69SPCh. 12 - Prob. 12.70SPCh. 12 - Prob. 12.71SPCh. 12 - Prob. 12.72SPCh. 12 - Prob. 12.73SPCh. 12 - Prob. 12.74SPCh. 12 - Prob. 12.75SPCh. 12 - Prob. 12.76SPCh. 12 - Prob. 12.77SPCh. 12 - Prob. 12.78SPCh. 12 - Prob. 12.79SPCh. 12 - Prob. 12.80SPCh. 12 - Prob. 12.81SPCh. 12 - Prob. 12.82SPCh. 12 - Prob. 12.83SPCh. 12 - Prob. 12.84SPCh. 12 - Prob. 12.85SPCh. 12 - Prob. 12.86SPCh. 12 - Prob. 12.87SPCh. 12 - Prob. 12.88SPCh. 12 - Prob. 12.89SPCh. 12 - Prob. 12.90SPCh. 12 - Prob. 12.91SPCh. 12 - Prob. 12.92SPCh. 12 - Prob. 12.93SPCh. 12 - Prob. 12.94SPCh. 12 - Prob. 12.95SPCh. 12 - Prob. 12.96SPCh. 12 - Prob. 12.97SPCh. 12 - Prob. 12.98SPCh. 12 - Prob. 12.99SPCh. 12 - Prob. 12.100SPCh. 12 - Prob. 12.101SPCh. 12 - A photovoltaic cell contains a p-n junction that...Ch. 12 - Prob. 12.103SPCh. 12 - Prob. 12.104SPCh. 12 - Prob. 12.105SPCh. 12 - Prob. 12.106SPCh. 12 - Prob. 12.107SPCh. 12 - Prob. 12.108SPCh. 12 - Prob. 12.109SPCh. 12 - Prob. 12.110SPCh. 12 - Prob. 12.111SPCh. 12 - Prob. 12.112SPCh. 12 - Prob. 12.113SPCh. 12 - Prob. 12.114SPCh. 12 - Prob. 12.115SPCh. 12 - Prob. 12.116SPCh. 12 - Prob. 12.117SPCh. 12 - Prob. 12.118SPCh. 12 - Prob. 12.119SPCh. 12 - Prob. 12.120SPCh. 12 - Prob. 12.121SPCh. 12 - Prob. 12.122SPCh. 12 - Prob. 12.123SPCh. 12 - Prob. 12.124SPCh. 12 - Prob. 12.125SPCh. 12 - Prob. 12.126SPCh. 12 - Prob. 12.127SPCh. 12 - Prob. 12.128SPCh. 12 - Prob. 12.129SPCh. 12 - Prob. 12.130SPCh. 12 - Prob. 12.131SPCh. 12 - Prob. 12.132SPCh. 12 - Prob. 12.133SPCh. 12 - Prob. 12.134MPCh. 12 - Prob. 12.135MPCh. 12 - Prob. 12.136MPCh. 12 - Prob. 12.137MPCh. 12 - Assume that 1588 g of an alkali metal undergoes...Ch. 12 - Prob. 12.139MPCh. 12 - Prob. 12.140MPCh. 12 - Prob. 12.141MPCh. 12 - Prob. 12.142MPCh. 12 - Prob. 12.143MPCh. 12 - Prob. 12.144MP
Knowledge Booster
Similar questions
- What is the product of the reaction shown below? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWrite the systematic name of each organic molecule: structure name П O ☐ O ☐ Oarrow_forwardThe 13C NMR signal for which of the indicated carbons will occur at the frequency (most deshielded)? Why is the correct answer E? Please explain what is happening. Please include a detailed explanation needed to understand the or question.arrow_forward
- Which of the following reagents best achieves the reaction shown below? Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the following reaction sequence? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardPls help ASAParrow_forward
- The reaction of phenylmagnesium bromide (C6H5MgBr) with propanal (CH3CH2CHO)3 followed by hydrolysis yields. A. 2-phenyl-1-propanol B. 1-phenyl-1propanol C. 3-phenyl-2-propanol D. 3-phenyl-1-propanol Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and/or a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the reaction sequence below? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question. The part that is under the pen in the image is (1) CH3CHO (2) H3O+arrow_forwardWhat is the missing reactant in this organic reaction? R+ OH HD CH3-CH2-CH-CH3 H A CH3 CH3-CH2-C-O-CH-CH2-CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No answer Click anywhere to draw the first atom of your structure. ☐ : Jm +arrow_forward
- Draw the major product of the following E2 reaction. Make sure you pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. Explain why this product is formed using 10 words or less for each. (a) NaH Br acetone TSO, NaH (b) acetonearrow_forward2. Circle the compound that will react SLOWER in an E2 reaction. To get credit for this question, you must EXPLAIN how you got your answer using STRUCTURES and WORDS. Br ** Br...arrow_forward8. 2 20 00 Draw ALL of the possible products for the following reaction CIRCLE the MAJOR product NaOMe MeOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
