
LCPO CHEMISTRY W/MODIFIED MASTERING
8th Edition
ISBN: 9780135214756
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.5P
Zinc sulfide crystallizes in the following cubic unit cell:
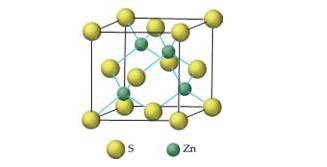
- How many sulfur ions and how many zinc ions are in each unit cell?
- What is the formula of zinc sulfide?
- What is the oxidation state of zinc?
- What is the geometry around each zinc atom?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the skeletal structure of the major organic product of each step of the reaction sequence.
Part: 0/2
Part 1 of 2
Part: 1/2
Part 2 of 2
Continue
OH
NaH
Na
Na
Br
+
Click and drag to start drawing a
structure.
X
:
X
G
:
G
please
please help me please please
Chapter 12 Solutions
LCPO CHEMISTRY W/MODIFIED MASTERING
Ch. 12 - Calcium metal crystallizes in a cubic...Ch. 12 - Polonium metal crystallizes in a simple cubic...Ch. 12 - Polonium metal crystallizes in a simple cubic...Ch. 12 - The density of a sample of metal "as measured to...Ch. 12 - Zinc sulfide crystallizes in the following cubic...Ch. 12 - Prob. 12.6ACh. 12 - Prob. 12.7PCh. 12 - Prob. 12.8ACh. 12 - Prob. 12.9PCh. 12 - Prob. 12.10A
Ch. 12 - Prob. 12.11PCh. 12 - Prob. 12.12ACh. 12 - Prob. 12.13PCh. 12 - Prob. 12.14PCh. 12 - Prob. 12.15PCh. 12 - Prob. 12.16PCh. 12 - Prob. 12.17PCh. 12 - Identify each of the following kinds of packingCh. 12 - Prob. 12.19CPCh. 12 - Titanium oxide crystallizes in the following cubic...Ch. 12 - Prob. 12.21CPCh. 12 - Prob. 12.22CPCh. 12 - Prob. 12.23CPCh. 12 - Prob. 12.24CPCh. 12 - Prob. 12.25CPCh. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - Prob. 12.29SPCh. 12 - Prob. 12.30SPCh. 12 - Prob. 12.31SPCh. 12 - Diffraction of X rays with =154.2 pm at an angle...Ch. 12 - Diffraction of X rays with =154.2 pm at an angle...Ch. 12 - Which of the four kinds of packing used by metals...Ch. 12 - What is a unit cell? How many atoms are in one...Ch. 12 - Copper crystallizes in a face-centered cubic unit...Ch. 12 - Lead crystallizes in a cubic unit cell with anedge...Ch. 12 - Prob. 12.38SPCh. 12 - Tungsten crystallizes in a body-centered cubic...Ch. 12 - Prob. 12.40SPCh. 12 - Prob. 12.41SPCh. 12 - Titanium metal has a density of and an atomic...Ch. 12 - Calcium metal has a density of 1.55 g/cm3 and...Ch. 12 - The atomic radius of Pb is 175 pm, and the density...Ch. 12 - The density of a sample of metal was measured to...Ch. 12 - If a protein can be induced to crystallize, its...Ch. 12 - The molecular structure of a scorpion toxin, a...Ch. 12 - Iron crystallizes in a body-centered cubic unit...Ch. 12 - Silver metal crystallizes in a face-centered cubic...Ch. 12 - Sodium hydride, NaH, crystallizes in a...Ch. 12 - Cesium chloride crystallizers in a cubic unit cell...Ch. 12 - If the edge length of an NaH unit cell is 488 pm,...Ch. 12 - The edge length of a CsCI unit cell (Problem...Ch. 12 - Silicon carbide, SiC, is a covalent network solid...Ch. 12 - Prob. 12.55SPCh. 12 - Prob. 12.56SPCh. 12 - Prob. 12.57SPCh. 12 - Prob. 12.58SPCh. 12 - Prob. 12.59SPCh. 12 - Prob. 12.60SPCh. 12 - Prob. 12.61SPCh. 12 - Prob. 12.62SPCh. 12 - Prob. 12.63SPCh. 12 - Prob. 12.64SPCh. 12 - Prob. 12.65SPCh. 12 - Prob. 12.66SPCh. 12 - Prob. 12.67SPCh. 12 - Prob. 12.68SPCh. 12 - Prob. 12.69SPCh. 12 - Prob. 12.70SPCh. 12 - Prob. 12.71SPCh. 12 - Prob. 12.72SPCh. 12 - Prob. 12.73SPCh. 12 - Prob. 12.74SPCh. 12 - Prob. 12.75SPCh. 12 - Prob. 12.76SPCh. 12 - Prob. 12.77SPCh. 12 - Prob. 12.78SPCh. 12 - Prob. 12.79SPCh. 12 - Prob. 12.80SPCh. 12 - Prob. 12.81SPCh. 12 - Prob. 12.82SPCh. 12 - Prob. 12.83SPCh. 12 - Prob. 12.84SPCh. 12 - Prob. 12.85SPCh. 12 - Prob. 12.86SPCh. 12 - Prob. 12.87SPCh. 12 - Prob. 12.88SPCh. 12 - Prob. 12.89SPCh. 12 - Prob. 12.90SPCh. 12 - Prob. 12.91SPCh. 12 - Prob. 12.92SPCh. 12 - Prob. 12.93SPCh. 12 - Prob. 12.94SPCh. 12 - Prob. 12.95SPCh. 12 - Prob. 12.96SPCh. 12 - Prob. 12.97SPCh. 12 - Prob. 12.98SPCh. 12 - Prob. 12.99SPCh. 12 - Prob. 12.100SPCh. 12 - Prob. 12.101SPCh. 12 - A photovoltaic cell contains a p-n junction that...Ch. 12 - Prob. 12.103SPCh. 12 - Prob. 12.104SPCh. 12 - Prob. 12.105SPCh. 12 - Prob. 12.106SPCh. 12 - Prob. 12.107SPCh. 12 - Prob. 12.108SPCh. 12 - Prob. 12.109SPCh. 12 - Prob. 12.110SPCh. 12 - Prob. 12.111SPCh. 12 - Prob. 12.112SPCh. 12 - Prob. 12.113SPCh. 12 - Prob. 12.114SPCh. 12 - Prob. 12.115SPCh. 12 - Prob. 12.116SPCh. 12 - Prob. 12.117SPCh. 12 - Prob. 12.118SPCh. 12 - Prob. 12.119SPCh. 12 - Prob. 12.120SPCh. 12 - Prob. 12.121SPCh. 12 - Prob. 12.122SPCh. 12 - Prob. 12.123SPCh. 12 - Prob. 12.124SPCh. 12 - Prob. 12.125SPCh. 12 - Prob. 12.126SPCh. 12 - Prob. 12.127SPCh. 12 - Prob. 12.128SPCh. 12 - Prob. 12.129SPCh. 12 - Prob. 12.130SPCh. 12 - Prob. 12.131SPCh. 12 - Prob. 12.132SPCh. 12 - Prob. 12.133SPCh. 12 - Prob. 12.134MPCh. 12 - Prob. 12.135MPCh. 12 - Prob. 12.136MPCh. 12 - Prob. 12.137MPCh. 12 - Assume that 1588 g of an alkali metal undergoes...Ch. 12 - Prob. 12.139MPCh. 12 - Prob. 12.140MPCh. 12 - Prob. 12.141MPCh. 12 - Prob. 12.142MPCh. 12 - Prob. 12.143MPCh. 12 - Prob. 12.144MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AG⁰ = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of N2 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm ☑ 5 00. 18 Ararrow_forwardi need help with the followingarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO(g) +Cl₂ (g) = 2NOC1 (g) AGº = -41. kJ Now suppose a reaction vessel is filled with 8.90 atm of chlorine (C12) and 5.71 atm of nitrosyl chloride (NOC1) at 1075. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. atm ☑ 18 Ararrow_forward
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCN is a weak acid. acids: 0.29 mol of NaOH is added to 1.0 L of a 1.2M HCN solution. bases: ☑ other: 0.09 mol of HCl is added to acids: 1.0 L of a solution that is bases: 0.3M in both HCN and KCN. other: 0,0,... ? 00. 18 Ar 日arrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: 0.2 mol of KOH is added to 1.0 L of a 0.5 M HF solution. bases: Х other: ☐ acids: 0.10 mol of HI is added to 1.0 L of a solution that is 1.4M in both HF and NaF. bases: other: ☐ 0,0,... ด ? 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. acids: ☐ 1.8 mol of HCl is added to 1.0 L of a 1.0M NH3 bases: ☐ solution. other: ☐ 0.18 mol of HNO3 is added to 1.0 L of a solution that is 1.4M in both NH3 and NH₁Br. acids: bases: ☐ other: ☐ 0,0,... ? 000 18 Ar B 1arrow_forward
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NH3 (g) = N2 (g) +3H₂ —N2 (g) AGº = 34. kJ Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NH 3 tend to rise or fall? ☐ x10 fall Х Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH 3 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no atm 00. 18 Ar 무ㅎ ?arrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 2.2 mol of NaOH is added to 1.0 L of a 1.4M HF solution. acids: П bases: Х other: ☐ ப acids: 0.51 mol of KOH is added to 1.0 L of a solution that is bases: 1.3M in both HF and NaF. other: ☐ 00. 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forward
- Homework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forwardIdentifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY