
(a)
Interpretation:
Considering the acid catalyzed mechanism of ester hydrolysis reaction answer the following,
The species which can be represented by
Concept Introduction:
An acid catalyzed hydrolysis of ester is much faster reaction as compared to uncatalyzed hydrolysis of ester. The acid catalyzed reaction mechanism is written as,
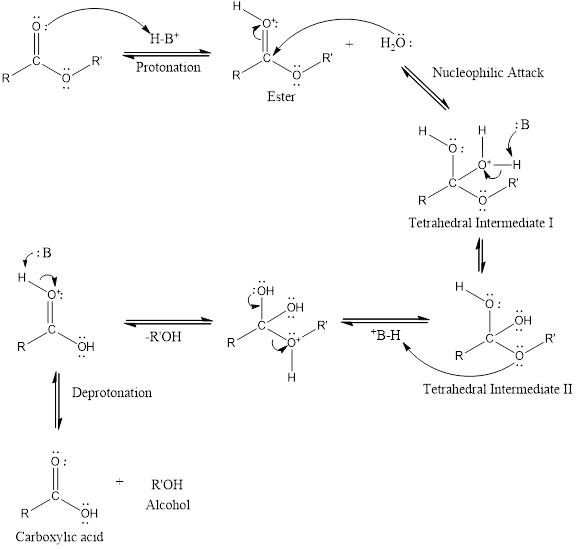
(b)
Interpretation:
Considering the acid catalyzed mechanism of ester hydrolysis reaction answer the following,
The species which can be represented by
Concept Introduction:
An acid catalyzed hydrolysis of ester is much faster reaction as compared to uncatalyzed hydrolysis of ester. The acid catalyzed reaction mechanism is written as,
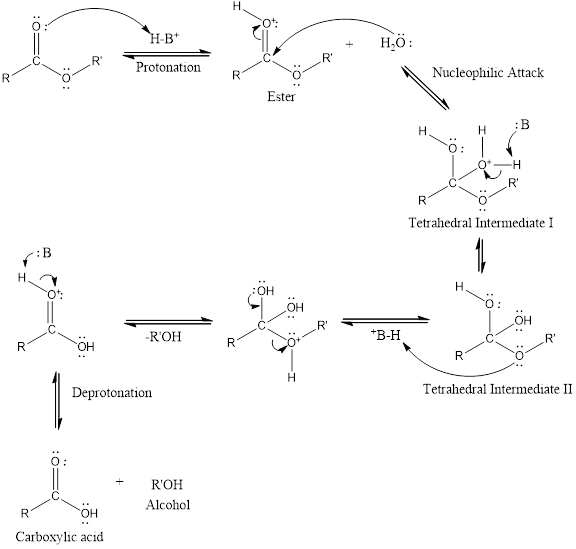
(c)
Interpretation:
Considering the acid catalyzed mechanism of ester hydrolysis reaction answer the following,
The species which is mostly used as
Concept introduction:
An acid catalyzed hydrolysis of ester is much faster reaction as compared to uncatalyzed hydrolysis of ester. The acid catalyzed reaction mechanism is written as,

(d)
Interpretation:
Considering the acid catalyzed mechanism of ester hydrolysis reaction answer the following,
The species which is mostly used as
Concept Introduction:
An acid catalyzed hydrolysis of ester is much faster reaction as compared to uncatalyzed hydrolysis of ester. The acid catalyzed reaction mechanism is written as,

Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- Show work.....don't give Ai generated solutionarrow_forwardShow work. Don't give Ai generated solutionarrow_forward10. Complete the following halogenation reactions for alkanes. Draw the structures of one of the many possible products for each reaction. Name the reactant and product. a) CH₂- CH-CH2-CH3 + Br₂ CH₂ UV UV b) + Cl2 c) CH3-CH₂ CHICHCHICH-CH CH₂-CH₂ + F2 UVarrow_forward
- Which of the following processes involves the largest photon energy? Group of answer choices Electron promotion from n=2 to n=5 Electron relaxing from n=4 to n=3 Ionization of an electron from n=2 Ionization of an electron from n=4arrow_forwardWhich of the following compounds does not match atomic ratio expectations in Mendeleev's 1872 periodic table? Group of answer choices NO2 Al2O3 SO3 CaOarrow_forwardNeed help with 14 and 15. 14. bromobenzene + (CHs),CuLi + THF / -78° followed by water quench is a. toluene else!! b. xylene c. cumene d. styrene e. something 15. When cumene + H,SO, / Na,Cr, 0,/water are mixed (refluxed) what is produced? a. 2-phenylpropanol phenol e. styrene b. benzoic acid c. no reaction!arrow_forward
- Which of the following orbitals intersect or overlap the x-axis in the standard cartesian coordinate system used? (Select ALL correct answers.) Group of answer choices px dxz dx2-y2 py dxy sarrow_forwardWhich of the following sets of elements is not a Dobereiner triad? (Choose the best answer.) Group of answer choices Li-Na-K Al-Ga-In Cr-Mo-W K-Rb-Csarrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardGive the structure(s) of the product(s) the reaction below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise I'm struggling to see how this reaction will go! I am wondering if it will cycle on itself but I'm not sure how I drew out a decagon but I'm a bit lostarrow_forwardGive the structure(s) of the product(s) for the reactions below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise .arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

