
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.3, Problem 5P
Muscalure is the sex attractant of the common housefly. Flies are lured to traps filled with bait that contain muscalure and an insecticide. Eating the bait is fatal. How could you synthesize muscalure using 1-bromopentane as one of the starting materials?
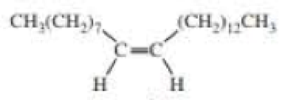
muscalure
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the following compound identify the lone pairs and indicate if each lone pair is localized or delocalized.
Please provide a thorough explanation that allows for undertanding of topic.
What is the relationship between the following compounds? Choose between: (a)constitutional isomers, (b)resonance structures, (c)identical, (d) conformers
Please provide a thorough explanation that allows for undertanding of topic.
Caffeine has the following structure. What is the hybridization state and molecular geometry at each nitrogen atom in Caffeine?
Please provide a thorough explanation that allows for undertanding of topic.
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY
Ch. 11.1 - Prob. 1PCh. 11.2 - Which is more reactive an organolithium compound...Ch. 11.2 - Prob. 3PCh. 11.3 - Muscalure is the sex attractant of the common...Ch. 11.3 - Prob. 7PCh. 11.3 - Prob. 8PCh. 11.3 - Prob. 9PCh. 11.3 - Prob. 10PCh. 11.4 - Prob. 13PCh. 11.4 - Prob. 14P
Ch. 11.4 - Prob. 15PCh. 11.4 - Prob. 16PCh. 11.4 - Prob. 17PCh. 11.4 - Prob. 19PCh. 11.4 - Show how the Suzuki and/or Heck reactions can be...Ch. 11.4 - Identify two pairs of an alkyl bromide and an...Ch. 11.5 - Prob. 22PCh. 11.5 - Draw the product of ring-closing metathesis for...Ch. 11.5 - Prob. 25PCh. 11.5 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - The coupling of an alkyne with an aryl halide in...Ch. 11 - Identify A through H.Ch. 11 - Using the given starting material, any necessary...Ch. 11 - What alkyl halide reacts with lithium...Ch. 11 - Prob. 33PCh. 11 - Prob. 34PCh. 11 - The following compound undergoes an intramolecular...Ch. 11 - Using ethynyleyclohexane as a starting material...Ch. 11 - Prob. 37PCh. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 39PCh. 11 - A student added an equivalent of...Ch. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 42PCh. 11 - Prob. 43PCh. 11 - Bombykol is the sex pheromone of the silk moth....Ch. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - A dibromide loses only one bromine when it reacts...Ch. 11 - What starting material is required in order to...Ch. 11 - What product is obtained from ring-opening...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forwardTryptophan is an essential amino acid important in the synthesis of neurotransmitter serotonin in the body. What are the hybridization states, molecular geometry and approximate bond angle at the indicated carbon and nitrogen atoms? Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardCan the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forward
- What are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forwardCan the following molecule be made in good yield from no more than two reactants, by moderately heating the reactants? If yes, draw the reactant or reactants. If no, then the product can't be made in one step.arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License