
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 49P
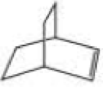
What product is obtained from ring-opening metathesis

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which will evaporate faster, 1-Butanol or Pentane? Explain your choice.
Using the equation below, what is the rate of this reaction if the rate of disappearance of H2 is 0.44 M/sec?
H2 + Br2 → 2HBr
2Fe3+(aq) + Sn2+(aq) □ 2Fe²+(aq) + Sn 4+ (aq)
If the change in Sn²+ concentration is 0.0010M in 38.5 seconds, what is the rate of disappearance of
Sn²+?
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY
Ch. 11.1 - Prob. 1PCh. 11.2 - Which is more reactive an organolithium compound...Ch. 11.2 - Prob. 3PCh. 11.3 - Muscalure is the sex attractant of the common...Ch. 11.3 - Prob. 7PCh. 11.3 - Prob. 8PCh. 11.3 - Prob. 9PCh. 11.3 - Prob. 10PCh. 11.4 - Prob. 13PCh. 11.4 - Prob. 14P
Ch. 11.4 - Prob. 15PCh. 11.4 - Prob. 16PCh. 11.4 - Prob. 17PCh. 11.4 - Prob. 19PCh. 11.4 - Show how the Suzuki and/or Heck reactions can be...Ch. 11.4 - Identify two pairs of an alkyl bromide and an...Ch. 11.5 - Prob. 22PCh. 11.5 - Draw the product of ring-closing metathesis for...Ch. 11.5 - Prob. 25PCh. 11.5 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - The coupling of an alkyne with an aryl halide in...Ch. 11 - Identify A through H.Ch. 11 - Using the given starting material, any necessary...Ch. 11 - What alkyl halide reacts with lithium...Ch. 11 - Prob. 33PCh. 11 - Prob. 34PCh. 11 - The following compound undergoes an intramolecular...Ch. 11 - Using ethynyleyclohexane as a starting material...Ch. 11 - Prob. 37PCh. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 39PCh. 11 - A student added an equivalent of...Ch. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 42PCh. 11 - Prob. 43PCh. 11 - Bombykol is the sex pheromone of the silk moth....Ch. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - A dibromide loses only one bromine when it reacts...Ch. 11 - What starting material is required in order to...Ch. 11 - What product is obtained from ring-opening...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For a neutral hydrogen atom with an electron in the n = 4 state, how many different energies are possible when a photon is emitted? 4 3 2 1 There are infinite possibilitiesarrow_forward2 NO(g) + H2(g) → N2(g) +2 H2O(g) If NO has rate of disappearance of 0.025 M/min, what is the rate of this reaction?arrow_forward2Fe3+(aq) + Sn2+(aq) □ 2Fe²+(aq) + Sn 4+ (aq) If the change in Sn2+ concentration is 0.0010M in 38.5 seconds, what is the rate of appearance of Fe²+?arrow_forward
- Using the equation below, if the rate of disappearance of Cl2 is 0.26 M/min, what is the rate of this reaction? 2NO(g) + Cl2(g) → 2NOCI(g)arrow_forwardA 45.0 mL solution containing a mixture of 0.0634 M KCN and 0.0634 M KCI is titrated with 0.107 M AgNO. From this mixture, which silver salt will precipitate first? A list of Ksp values can be found in the table of solubility constants. • AgCI • not enough information to determine AgCN What is the concentration of Ag* at the first equivalence point? [Ag*] = Will the second silver salt begin to precipitate at the first equivalence point before the first silver salt has completely precipitated? • not enough information to determine • yes • noarrow_forward[Review Topics] [References] Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the same compound, or the same conformation of a compound viewed from a different perspective. Note that cis, trans isomers are an example of stereoisomers. H₂N ✓ CI H₂N NH2 NH₂ CI Submit Answer Retry Entire Group 2 more group attempts remaining Previous Next>arrow_forward
- Don't used Ai solutionarrow_forwardDraw resonance structures for the following compounds. Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardBF3 has a no dipole moment. a) Draw the Lewis structure for BF3, showing all nonbonding electrons. b) Indicate the polarity of every atom in the structure using δ+ and δ– notation, and explain why the molecule has no net dipole. Please provide a thorough explanation that allows for undertanding of topic.arrow_forward
- For each reaction shown below follow the curved arrows to complete each equation by showing the structure of the products. Identify the acid, the base, the conjugated acid and conjugated base. Consutl a pKa table and choose the direciton the equilibrium goes. Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardNeed help understanding please help Let’s assume the initial volume of the gas is 4.80 LL , the initial temperature of the gas is 29.0 °C°C , and the system is in equilibrium with an external pressure of 1.2 bar (given by the sum of a 1 bar atmospheric pressure and a 0.2 bar pressure due to a brick that rests on top of the piston). What is the final pressure of the gas? What is the final volume of the gas? What happens with the piston after you finish heating the gas? Assume you do not need to worry about the gas cooling down again because the outside of the container is at a lower temperature. That is, you manage to keep the gas at a constant temperature that equals 54.2 °C°C What is the sign of w? What is the value of w? Be careful with units. How do you convert bar*L to J?arrow_forwardFor a neutral hydrogen atom with an electron in the n = 4 state, how many different energies are possible when a photon is emitted?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY