
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.11, Problem 19P
Draw the products of each reaction.
a. 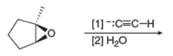 b.
b.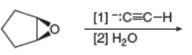
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider this molecule:
How many H atoms are in this molecule?
How many different signals could be found in its 1H NMR spectrum?
Note: A multiplet is considered one signal.
For each of the given mass spectrum data, identify whether the compound contains chlorine, bromine, or neither.
Compound
m/z of M* peak
m/z of M
+ 2 peak
ratio of M+ : M
+ 2 peak
Which element is present?
A
122
no M
+ 2 peak
not applicable
(Choose one)
B
78
80
3:1
(Choose one)
C
227
229
1:1
(Choose one)
Show transformation from reactant to product, step by step. *see image
Chapter 11 Solutions
Organic Chemistry (6th Edition)
Ch. 11.1 - Problem 11.1 Neopheliosyne B is a novel acetylenic...Ch. 11.2 - Give the IUPAC name for each compound.Ch. 11.2 - Give the structures corresponding to each of the...Ch. 11.3 - Prob. 4PCh. 11.5 - Prob. 5PCh. 11.6 - Which bases can deprotonate acetylene? The pKa...Ch. 11.7 - Draw the organic products formed when each alkyne...Ch. 11.7 - Draw additional resonance structures for each...Ch. 11.8 - Problem 11.9 Draw the products formed when is...Ch. 11.8 - Explain the following result. Although alkenes...
Ch. 11.9 - Problem 11.11 Draw the keto tautomer of each...Ch. 11.9 - Prob. 12PCh. 11.9 - a Draw two different enol tautomers of...Ch. 11.10 - Prob. 14PCh. 11.10 - Problem 11.15 Draw the organic products formed in...Ch. 11.11 - Problem 11.16 What acetylide anion and alkyl...Ch. 11.11 - Problem. 11.17 Show how , and can be used to...Ch. 11.11 - Prob. 18PCh. 11.11 - Draw the products of each reaction. a. b.Ch. 11.11 - Prob. 20PCh. 11 - Prob. 25PCh. 11 - 11.25 Answer the following questions about...Ch. 11 - 11.26 Give the IUPAC name for each alkyne.
a. ...Ch. 11 - Prob. 28PCh. 11 - Which of the following pairs of compounds...Ch. 11 - Prob. 30PCh. 11 - 11.30 How is each compound related to A? Choose...Ch. 11 - Prob. 32PCh. 11 - 11.33 Draw the products formed when is treated...Ch. 11 - What reagents are needed to convert (CH3CH2)3CCCH...Ch. 11 - 11.36 What alkynes give each of the following...Ch. 11 - 11.37 What alkyne gives each compound as the only...Ch. 11 - 11.38 Draw the organic products formed in each...Ch. 11 - 11.42 What reactions are needed to convert alcohol...Ch. 11 - 11.50 What acetylide anion and alkyl halide are...Ch. 11 - 11.52 Devise a synthesis of each compound using ...Ch. 11 - Prob. 58PCh. 11 - 11.59 N-Chlorosuccinimide (NCS) serves as a source...Ch. 11 - 11.60 Draw a stepwise mechanism for the following...Ch. 11 - 11.61 Draw a stepwise mechanism for the following...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
The validity of a scientific law.
Physical Universe
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forwardE17E.2(a) The following mechanism has been proposed for the decomposition of ozone in the atmosphere: 03 → 0₂+0 k₁ O₁₂+0 → 03 K →> 2 k₁ Show that if the third step is rate limiting, then the rate law for the decomposition of O3 is second-order in O3 and of order −1 in O̟.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY