
Concept explainers
Answer the following questions about erlotinib and phomallenic acid C. Erlotinib, sold under the trade name Tarceva, was introduced in 2004 for the treatment of lung cancer. Phomallenic acid C is an inhibitor of bacterial fatty acid synthesis.
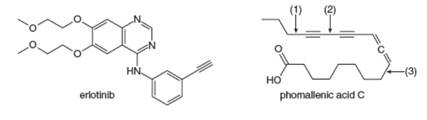
a. Which
b. What orbitals are used to form the shortest
c. Which H atom in phomallenic acid C is most acidic.
d. How many
e. Rank the labeled bonds in phomallenic acid C in order of increasing bond strength.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Organic Chemistry (6th Edition)
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Fundamentals Of Thermodynamics
Human Biology: Concepts and Current Issues (8th Edition)
HUMAN ANATOMY
- For the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forwardidentify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: ? or CI CI 1) NaNHz (excess) 2) H₂O OA OB O C OD OE OF H₂SO₂, H₂O Hq50. 1) n-BuLi 2) Br 1) 9-BBN 2) H₂O₂, NaOH A B H H متته D E H H H H C H H F H H H₂ Lindlar's catalyst Na NHarrow_forward
- Identify the product (or multiple products) for each of the following reactions: O A OB Oc OD OE OF CI CI 1) NaNH2 (excess) 2) H₂O H₂ H₂SO2, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) Br 1)9-BBN 2) H₂O₂, NaOH ? Na, NH3 C H A H H مننه مننه منن مننه H F H H E مند H D H Harrow_forwardFor the following compound: HO H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: + H-O: H-OH₂ H Mechanism B: H-Ö: HO-H H-OO -H H HH H H HH H-O: H-OO H-OO -H H e -H : OH Θ Mechanism C: Θ A : OH H-O: H H H-O-H 0. Mechanism D: e.. : OH :0 H H-O-H H-O: H-OO :O H -H H H сём H 0 :0 + H Θ H H H-arrow_forwardFor the following compound: H OH Draw a mechanism for the tautomerization process under ACIDIC conditions: Mechanism A: Θ :OH O O-H HO 0: Mechanism B: :O-H e.. Θ :OH Mechanism C: H HO-H :0: Θ 0: H H e.. : OH 0: "Θ HH O. :OH :OH O-H O-H Mechanism D: :OH H-OH₂ :OH HO-H 0: © O-H H HH 0: HHarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



