
Concept explainers
The process that causes the greatest amount of exergy destruction.
Answer to Problem 117RP
The process that causes the greatest amount of exergy destruction is
Explanation of Solution
Show the T-s diagram for refrigeration system as in Figure (1).
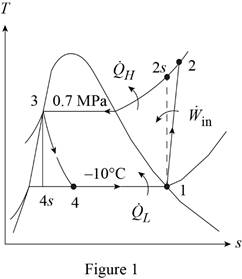
From Figure (1), write the specific enthalpy at state 3 is equal to state 4 due to throttling process.
Here, specific enthalpy at state 3 and 4 is
Express specific enthalpy at state 2 using compressor efficiency.
Here, specific enthalpy at state 2, 1 and 2s is
Express temperature at state 3.
Here, saturation temperature at pressure of
Express quality at state 4.
Here, specific enthalpy at saturation liquid and temperature of
Express specific entropy at state 4.
Here, specific entropy at saturation liquid and temperature of
Express heat rejected in the evaporator.
Here, specific enthalpy at state 4 is
Express heat added in the condenser.
Express the exergy destruction during process 1-2.
Here, surrounding temperature is
Express the exergy destruction during process 2-3.
Here, specific entropy at state 3 is
Express the exergy destruction during process 3-4.
Here, specific entropy at state 4 is
Express the exergy destruction during process 4-1.
Here, freezing temperature of water is
Conclusion:
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the properties corresponding to initial temperature
Here, specific entropy at state 1 is
Perform unit conversion of pressure at state 2 from
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 2s corresponding to pressure at state 2 of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is specific entropy at state 2 and specific enthalpy at state 2s respectively.
Show the specific enthalpy at state 2s corresponding to specific entropy as in Table (1).
|
Specific entropy at state 2 |
Specific enthalpy at state 2s |
| 0.9314 | 268.47 |
| 0.9378 | |
| 0.9642 | 278.59 |
Substitute
Thus, the specific enthalpy at state 2s is,
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the saturated temperature corresponding to pressure of
Substitute
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the specific enthalpy and entropy at state 3 corresponding to temperature at state 3
Here, specific enthalpy and entropy at saturated liquid is
Substitute
Substitute
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the properties corresponding to final temperature
Substitute
Substitute
Refer Table A-13, “superheated refrigerant 134a”, and write the specific entropy at state 2 corresponding to pressure at state 2 of
Show the specific entropy at state 2 corresponding to specific enthalpy as in Table (2).
|
Specific enthalpy at state 1 |
Specific entropy at state 2 |
| 268.47 | 0.9314 |
| 275.02 | |
| 278.59 | 0.9642 |
Use excels and tabulates the values from Table (2) in Equation (XII) to get,
Substitute
Substitute
Perform unit conversion of temperature from
Substitute
Substitute
Substitute
Take the freezing temperature of water as,
Substitute
Hence, the process that causes the greatest amount of exergy destruction is
Want to see more full solutions like this?
Chapter 11 Solutions
Thermodynamics: An Engineering Approach ( 9th International Edition ) ISBN:9781260092684
- Correct answer is written below. Detailed and complete solution only with fbd. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forward
- Correct answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





