
Concept explainers
Interpretation:
The density of a hypothetical ionic compound for given radii and masses of cations and anions, is to be determined.
Concept introduction:
Ionic compounds contain cations and anions that are arranged in a crystal lattice.
A crystal lattice is made of small repeating unit cells.
A unit cell is of primitive or centered type.
Each atom or ion in a unit cell is shared by the adjacent cells. An atom or ions at corners is shared by eight unit cells.
The edge length of a body-centered cubic cell is given by the relation as follows:
Here,
is the edge length,
is the radius of cation, and
The volume of the unit cell
The density
Here,
is the mass.
Answer to Problem 146AP
Solution:
Explanation of Solution
Given information:
Radii of the anion
The figure is as shown below:
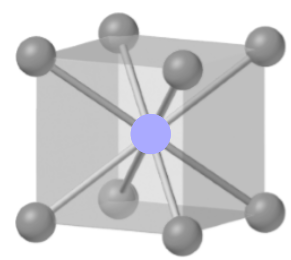
The given unit cell is body-centered, in which there is one cation at the center, and eight anions at the corners, shared by eight unit cells.
The contribution of 8 anions at the corners to one unit cell is:
In the unit cell, one anion and one cation are present.
The mass of an anion is
and that of a cation is
The relation between
and
is as:
Convert the mass to grams as follows:
Similarly,
Thus, the mass of the unit cell will be:
The formula to calculate the edge length is as:
Substitute
for
and
for
in the above expression as:
Convert the edge length to
as follows:
The expression to calculate volume is as:
Substitute
in the above expression as:
Now, finally calculate density as:
Substitute
for
for
in the above expression as:
The density of the hypothetical ionic compound is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry
- What is the missing reactant R in this organic reaction? N N H3O+ +R + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure. fmarrow_forwardThe product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH3CN H+ N Click and drag to start drawing a structure. 5arrow_forwardAssign this HSQC Spectrum ( please editing clearly on the image)arrow_forward
- (a 4 shows scanning electron microscope (SEM) images of extruded actions of packing bed for two capillary columns of different diameters, al 750 (bottom image) and b) 30-μm-i.d. Both columns are packed with the same stationary phase, spherical particles with 1-um diameter. A) When the columns were prepared, the figure shows that the column with the larger diameter has more packing irregularities. Explain this observation. B) Predict what affect this should have on band broadening and discuss your prediction using the van Deemter terms. C) Does this figure support your explanations in application question 33? Explain why or why not and make any changes in your answers in light of this figure. Figure 4 SEM images of sections of packed columns for a) 750 and b) 30-um-i.d. capillary columns.³arrow_forwardfcrip = ↓ bandwidth Il temp 32. What impact (increase, decrease, or no change) does each of the following conditions have on the individual components of the van Deemter equation and consequently, band broadening? Increase temperature Longer column Using a gas mobile phase instead of liquid Smaller particle stationary phase Multiple Paths Diffusion Mass Transferarrow_forward34. Figure 3 shows Van Deemter plots for a solute molecule using different column inner diameters (i.d.). A) Predict whether decreasing the column inner diameters increase or decrease bandwidth. B) Predict which van Deemter equation coefficient (A, B, or C) has the greatest effect on increasing or decreasing bandwidth as a function of i.d. and justify your answer. Figure 3 Van Deemter plots for hydroquinone using different column inner diameters (i.d. in μm). The data was obtained from liquid chromatography experiments using fused-silica capillary columns packed with 1.0-μm particles. 35 20 H(um) 큰 20 15 90 0+ 1500 100 75 550 01 02 594 05 μ(cm/sec) 30 15 10arrow_forward
- elow are experimentally determined van Deemter plots of column efficiency, H, vs. flow rate. H is a quantitative measurement of band broadening. The left plot is for a liquid chromatography application and the night is for gas chromatography. Compare and contrast these two plots in terms of the three band broadening mechanisms presented in this activity. How are they similar? How do they differ? Justify your answers.? 0.4 H (mm) 0.2 0.1- 0.3- 0 0.5 H (mm) 8.0 7.0 6.0 5.0 4.0- 3.0 T +++ 1.0 1.5 0 2.0 4.0 Flow Rate, u (cm/s) 6.0 8.0 Flow Rate, u (cm/s)arrow_forwardPredict the products of this organic reaction: + H ZH NaBH3CN H+ n. ? Click and drag to start drawing a structure. Xarrow_forwardWhat is the missing reactant R in this organic reaction? + R H3O+ + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure.arrow_forward
- What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardThe product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH₂CN H+ N Click and drag to start drawing a structure. X $arrow_forwardExplain what is the maximum absorbance of in which caffeine absorbs?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





