
Concept explainers
(a)
Interpretation:
The condensed formula of
Concept Introduction:
Condensed structural formula: Condensed structural formula shows the arrangement of atoms in grouped form.
(a)
Answer to Problem 11A.12E
The condensed formula of
Explanation of Solution
The given compound is,
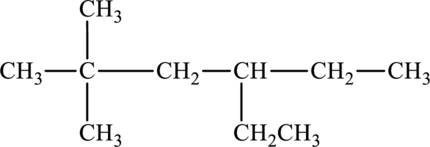
The parent chain of the compound is hexane. One ethyl group is present in the carbon fourth position and two methyl groups are present at carbon second position. Hence, the systematic name of the compound is
(b)
Interpretation:
The condensed formula of
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 11A.12E
The condensed formula of
Explanation of Solution
The given compound is,
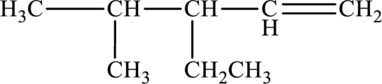
The parent chain of the compound is pentane. One ethyl group is present in the carbon third position and one methyl group is present in carbon fourth position. One double bond is present at the carbon first position.
Hence, the systematic name of the compound is
(c)
Interpretation:
The condensed formula of
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 11A.12E
The condensed formula of
Explanation of Solution
The given compound is,
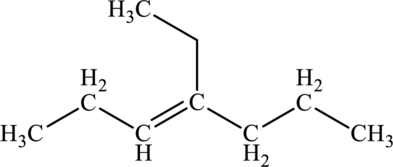
The parent chain of the compound is heptane. One methyl group is present in carbon fourth position and a double bond is seen at the carbon third position. Hence, the systematic name of the compound is
(d)
Interpretation:
The condensed formula of
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 11A.12E
The condensed formula of
Explanation of Solution
The given compound is,
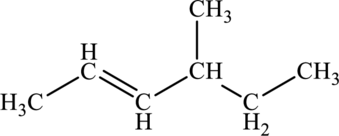
The parent chain of the compound is hexane. One methyl group is present in the carbon fourth position and a double bond is present in carbon second position.
Hence, the systematic name of the compound is
Want to see more full solutions like this?
Chapter 11 Solutions
CHEMICAL PRINCIPLES (LL) W/ACCESS
- Using iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forwardDraw the product formed when the following pair of compounds is treated with NaOEt in ethanol. + i CNarrow_forwardI need help with the followingarrow_forward
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
