
(a)
Interpretation:
The systematic name of the compound
Concept Introduction:
Rules for naming compound:
- 1) The carbon atoms in the longest chain have to be counted.
- 2) The substituents and multiple bonds have to be identified and counted and the suffix “-ene” is added.
- 3) The backbone carbon atoms have to be numbered by assigning the lowest number from the starting end that contains the double bond.
Rules for naming compound:
- 1) The carbon atoms in the longest chain have to be counted.
- 2) The substituents and multiple bonds have to be identified and counted and the suffix “-yne” is added.
- 3) The backbone carbon atoms have to be numbered by assigning the lowest number from the starting end that contains the double bond.
Geometrical isomers: In geometrical isomers, atoms have different arrangements on either side of a double bond above or below the ring of a cycloalkane or cycloalkane. If the atoms are present on the same side of the double bond, then it is cis-isomer and if they are present on the opposite side of the double bond, then it is trans-isomer.
(a)
Answer to Problem 11.8E
The systematic name of the compound
Cis-
Explanation of Solution
The given compound is,
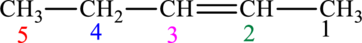
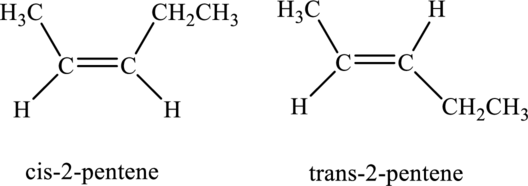
The compound is identified as alkene. The parent chain of the compound is pentane and a double bond is seen at the carbon second position. Hence, the systematic name of the compound is
Cis-
(b)
Interpretation:
The systematic name of the compound
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 11.8E
The systematic name of the compound
The geometrical isomers are not possible in
Explanation of Solution
The given compound is,
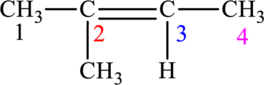
The compound is identified as alkene. The parent chain of the compound is butane. One methyl group is present in the carbon second position and a double bond is seen at the carbon second position. Hence, the systematic name of the compound is
No geometrical isomers are possible in
(c)
Interpretation:
The systematic name of the compound
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 11.8E
The systematic name of the compound
No geometrical isomers are possible in
Explanation of Solution
The given compound is,
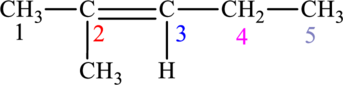
The compound is identified as alkene. The parent chain of the compound is pentane. One methyl group is present in the carbon second position and a double bond is seen at the carbon second position. Hence, the systematic name of the compound is
No geometrical isomers are possible in
(d)
Interpretation:
The systematic name of the compound
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 11.8E
The systematic name of the compound
No geometrical isomers are possible in
Explanation of Solution
The given compound is,
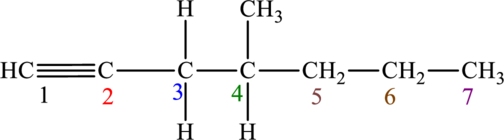
The compound is identified as alkyne. The parent chain of the compound is heptane. One triple bond is present in carbon first position and one methyl substituent is present in carbon fourth position. Hence, the systematic name of the compound is
Geometrical isomers are not possible because triple bond has only one substituent each.
(e)
Interpretation:
The systematic name of the compound
Concept Introduction:
Refer to part (a).
(e)
Answer to Problem 11.8E
The systematic name of the compound
No geometrical isomers are possible in
Explanation of Solution
The given compound is,
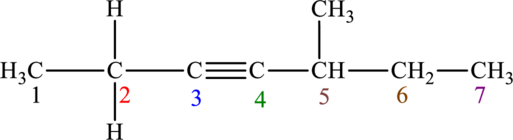
The compound is identified as alkyne. The parent chain of the compound is heptane. One triple bond is present in carbon third position and one methyl substituent is present in carbon fifth position. Hence, the systematic name of the compound is
Geometrical isomers are not possible because triple bond has only one substituent each.
Want to see more full solutions like this?
Chapter 11 Solutions
CHEMICAL PRINCIPLES (LL) W/ACCESS
- 1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
- Predict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward
- 个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

