
Concept explainers
(a)
Interpretation:
The structural formula of the product of the reaction of glycerol with stearic acid has to be drawn.
Concept Introduction:
Preparation of Esters:
Esters are prepared from the reaction of
The general preparation of esters is shown below,
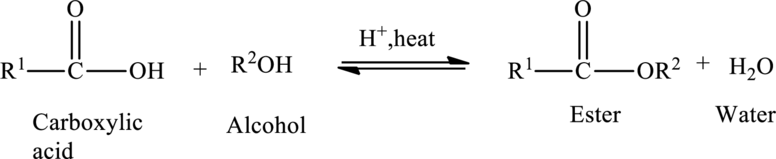
Structural formula: Structural formulas identify the location of
(b)
Interpretation:
The structural formula of the product of the oxidation reaction of 4-hydroxybenzyl alcohol has to be drawn.
Concept Introduction:
The oxidation of a primary alcohol produces an aldehyde.
The generalized equation is written as,
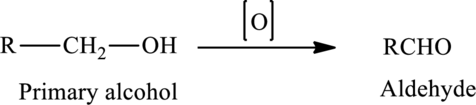
The oxidation of a secondary alcohol produces a ketone.
The generalized equation is written as,
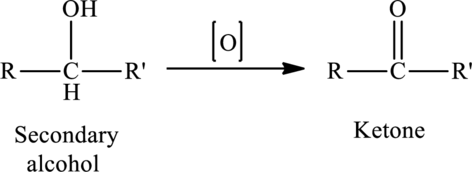
Structural formula: Structural formulas identify the location of chemical bonds between the atoms of a molecule. A structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds-one, two or three standing lines for single, double or triple bonds respectively.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
CHEMICAL PRINCIPLES (LL) W/ACCESS
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

