
(a)
Interpretation:
IUPAC name has to be provided and equation has to be written for hydration of the given molecule.
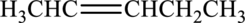
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming
There are about five rules that has to be followed for naming an alkene and an
- The longest continuous carbon chain in the compound that contains double bond or triple has to be identified. This is known as parent compound.
- Suffix “–ane” (in name of
alkane ) is replaced with “-ene” for alkene or “-yne” for alkyne. - Numbering has to be done so that the lowest number is given to the double or triple bond.
- Naming and numbering has to be given for each atom or group that is attached to the parent chain. Numbering has to be done in a way that substituents get the least numbering.
- If the alkenes have more than one double bond they are called as alkadienes (two double bonds) or alkatrienes (three double bonds). Appropriate suffix has to be used depending on the number of multiple bonds present in the compound.
Hydration of alkene:
Addition of water molecule to an unsaturated bond present in hydrocarbon is known as hydration. During hydration, the unsaturated bond present is broken and a carbon‑hydrogen, carbon‑hydroxyl bond is formed.
General structure of alkene can be given as,

Alkene on hydration gives alcohol. As there is only one unsaturated bond present in alkene, only one molecule of water is consumed. The general equation for the hydration of alkene can be given as,
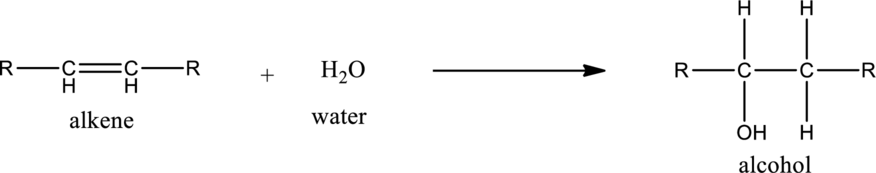
If the alkene is symmetrical only one product is formed. If the alkene is unsymmetrical, then the product is formed according the Markovnikov’s rule. According to this rule, in hydration reaction, the hydrogen atom gets attached to the carbon atom in the unsaturated bond which has more number of hydrogen atoms or less number of substituents.
(b)
Interpretation:
IUPAC name has to be provided and equation has to be written for hydration of the given molecule.
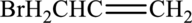
Concept Introduction:
Refer part (a).
(c)
Interpretation:
IUPAC name has to be provided and equation has to be written for hydration of the given molecule.
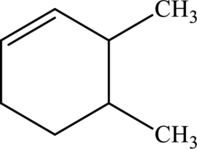
Concept Introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY-ALEKS 360
- H3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forward
- Is the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forward
- If CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward+ Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward+ Provide the correct IUPAC name for the compound shown here. Reset H3C H H C CH3 CH-CH3 1-3-methylpent ene trans- cis- 5-6-3-1-2-4- tert- tri sec- di cyclo iso but pent hex meth prop eth yl ane ene yne ☑arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





