
Concept explainers
(a)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the
Answer to Problem 11.77AP
The curved arrow mechanism for the reaction is shown below.
Explanation of Solution
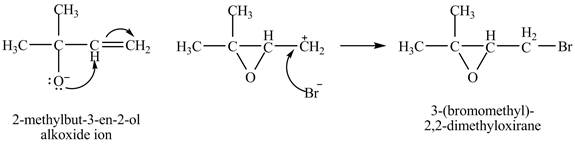
The given reaction is shown below.
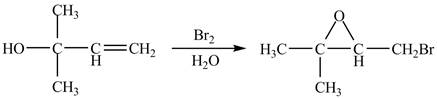
Figure 1
The oxygen atom of the alkoxide ion attack carbon of double bond to form

Figure 2
The curved arrow mechanism for given reaction is shown in Figure 2.
(b)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism for the given reaction is shown below.
Explanation of Solution
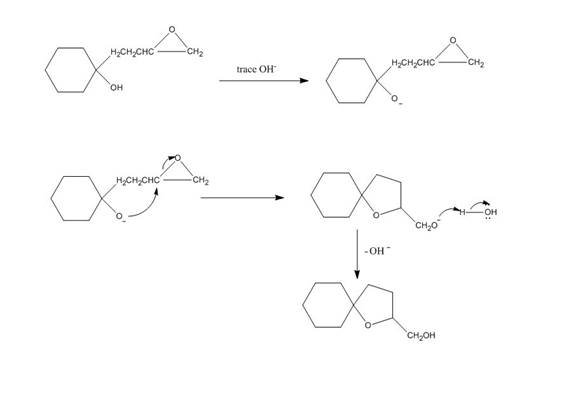
The given reaction is shown below.
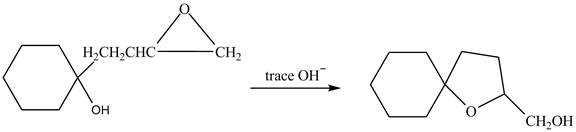
Figure 3
Hydroxide ion abstract the proton from hydroxyl group of given compound. The oxide attacks the carbon of epoxide ring to form a five membered ring. The alkoxide ion formed is protonated in presence of water. The curved arrow mechanism of the given reaction is shown below.
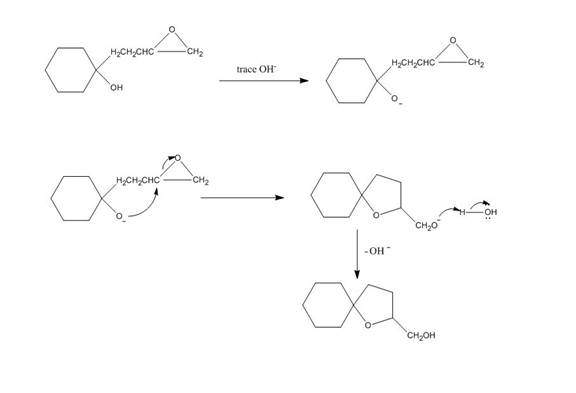
Figure 4
The curved arrow mechanism for given reaction is shown in Figure 4.
(c)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
Explanation of Solution
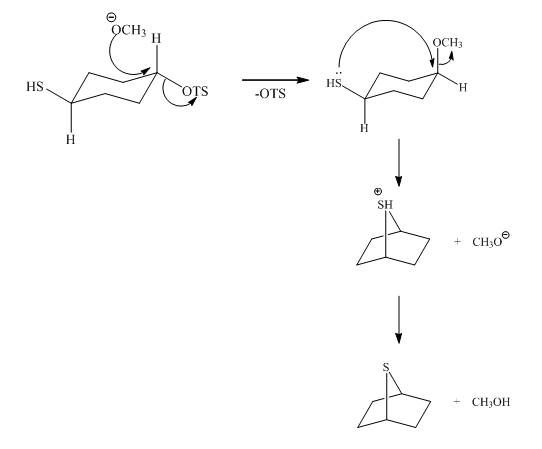
The given reaction is shown below.
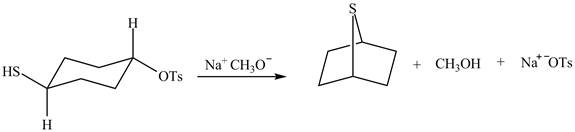
Figure 5
The methoxy group attacks the carbon containing sulfonate ester group. The nucleophilic displacement reaction occurs. The sulfonate ester group is displaced by methoxy group. Then neighbouring group participation of sulfur atom displaces the methoxy group. The sulfonium ion formed undergoes deprotonation to form the product. The curved arrow mechanism of the given reaction is shown below.

Figure 6
The curved arrow mechanism for given reaction is shown in Figure 6.
(d)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
Explanation of Solution
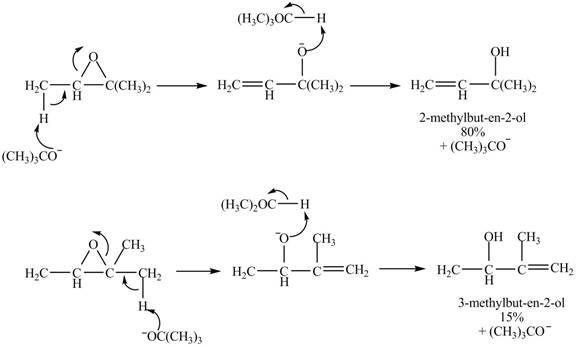
The given reaction is shown below.
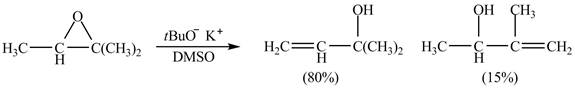
Figure 7
A strong base is used in the reaction; hence elimination reaction will take place. The given compound contains two
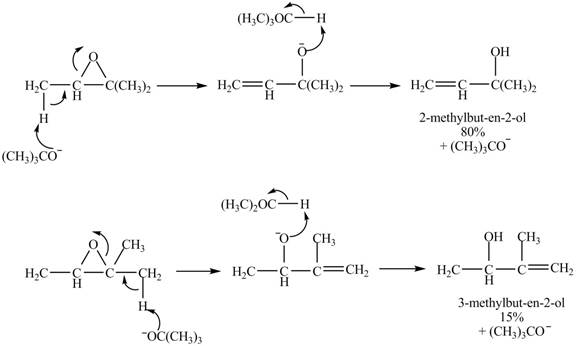
Figure 8
The curved arrow mechanism for given reaction is shown in Figure 8.
(e)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
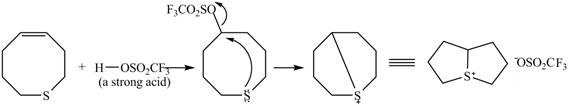
Explanation of Solution
The given reaction is shown below.
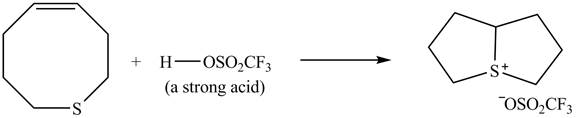
Figure 9
The
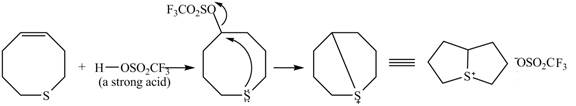
Figure 10
The curved arrow mechanism for given reaction is shown in Figure 10.
(f)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
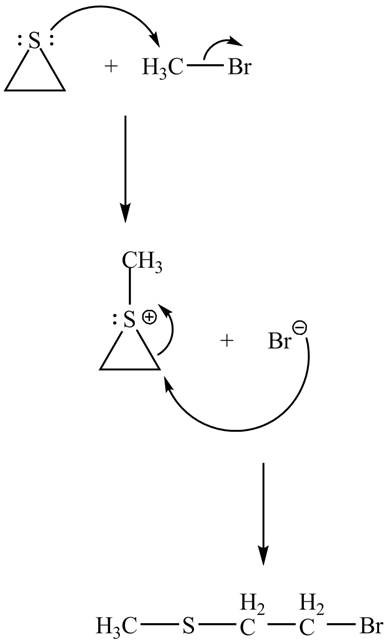
Explanation of Solution
The given reaction is shown below.
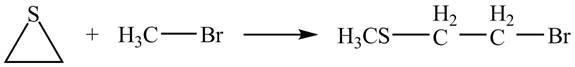
Figure 11
The lone pair of sulphur atom attacks the carbon of methyl bromide. The sulfur atom of the ring gets methylated. Then bromide ion attacks the carbon atom of ring which results in ring opening to form the desired product. The curved arrow mechanism of the given reaction is shown below.

Figure 12
The curved arrow mechanism for given reaction is shown in Figure 12.
(g)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
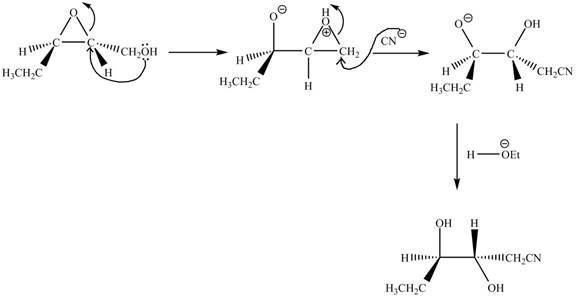
Explanation of Solution
The given reaction is shown below.
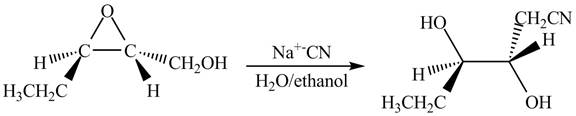
Figure 13
The neighbouring group participation of oxygen atom takes place in the given reaction. The oxygen atom of hydroxyl group attacks the electrophilic carbon atom of the epoxide ring which results in the formation of new epoxide ring. Then cyanide ion attacks at the electrophilic carbon of epoxide which results in ring opening. The oxide ion takes proton form ethanol to form desired product. The curved arrow mechanism for given reaction is shown below.

Figure 14
The curved arrow mechanism for given reaction is shown in Figure 14.
(h)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
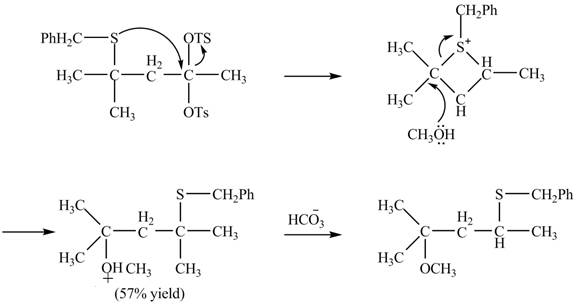
Explanation of Solution
The given reaction is shown below.
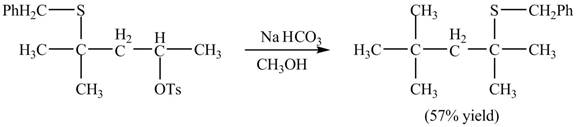
Figure 15
The sulfur atom attacks at the electrophilic carbon atom to form a four membered ring. The nucleophilic attack by methanol at the ring results in ring opening. Then the base abstracts proton from the protonated hydroxyl group to form the desired product. The curved-arrow mechanism for the given reaction is shown below.
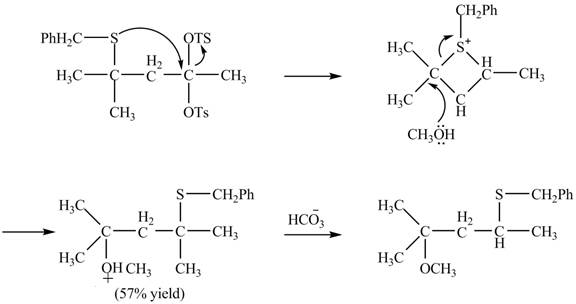
Figure 16
The curved arrow mechanism for given rearrangement is shown in Figure 16.
(i)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
The vicinal
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
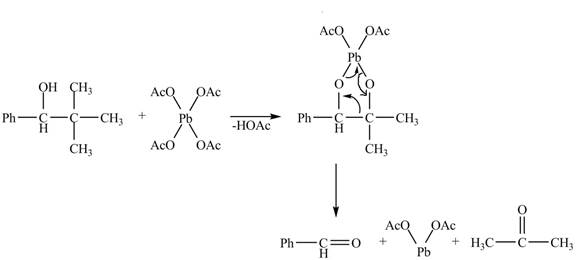
Explanation of Solution
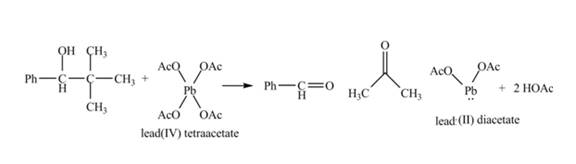
Figure 17
The given compound is a vicinal diol. It will react with the lead tetraacetate to form five membered ring intermediate. The intermediate formed rearranges to form acetone, benzaldehyde and lead diacetate. The curved arrow mechanism for the given reaction is shown below.
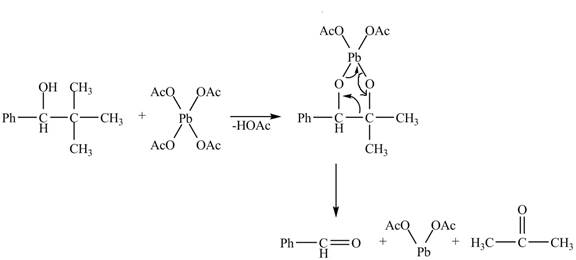
Figure 18
The curved arrow mechanism for given reaction is shown in Figure 18.
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry Study Guide and Solutions
- Predict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forwardPlease draw the structuresarrow_forwardDraw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forward
- Draw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forwardWhat is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forward
- What is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward> aw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 1. Eto 1. EtO¯ H3O+ 1 2 2. PrBr 2. PrBr Δ You can draw the three structures in any arrangement you like. 3 Click and drag to start drawing a structure. Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacarrow_forward
- There are various factors that affect an equilibrium. Give 3 of these factors and explain using examples andequations how an equilibrium is affected by these factors. Please remember that this is a communication question so that you are communicating your understanding of the factors that affect and equilibrium.arrow_forwardEEZE LETCHUP ID Draw the most likely conjugate base resulting from this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. Drawing く NaOCH2CH3 :0: :0: 狗arrow_forwardAnswerarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

