
(a)
Interpretation:
State that the copper element A or element B is labeled in given phase diagram of copper-silver metals.
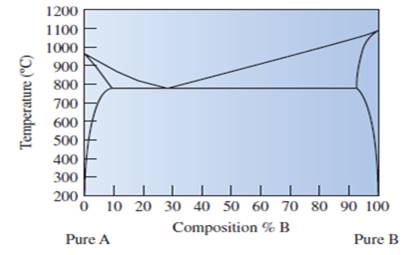
Fig.
Concept Introduction:
The properties of the copper-silver alloy are as follows-
1. Melting point −
2. Molecular weight −
3. Density −
4. Insoluble in water
In the case of silver and copper alloy, both of these are not able to melt together and form a new alloy with
1. Sterling silver − In sterling silver, the weight percentage of pure silver is
2. Coin silver - The weight percentage of pure silver available in coin silver is
There is a new alloy also available known as Electrum. In this alloy, the weight percentage of silver is from
(b)
Interpretation:
The well labelled phase diagram of the copper silver alloy needs to be sketched.
Concept Introduction:
The properties of the copper-silver alloy are as follows-
1. Melting point −
2. Molecular weight −
3. Density −
4. Insoluble in water
In the case of silver and copper alloy, both of these are not able to melt together and form a new alloy with
1. Sterling silver − In sterling silver, the weight percentage of pure silver is
2. Coin silver - The weight percentage of pure silver available in coin silver is
There is a new alloy also available known as Electrum. In this alloy, the weight percentage of silver is from
(c)
Interpretation:
Whether the new composition is stronger or weaker needs to be determined, if it cools down at
Concept Introduction:
The properties of the copper-silver alloy are as follows-
1. Melting point −
2. Molecular weight −
3. Density −
4. Insoluble in water
In the case of silver and copper alloy, both of these are not able to melt together and form a new alloy with
1. Sterling silver − In sterling silver, the weight percentage of pure silver is
2. Coin silver - The weight percentage of pure silver available in coin silver is
There is a new alloy also available known as Electrum. In this alloy, the weight percentage of silver is from
(d)
Interpretation:
The statement "microstructure can lead to the discrepancy" needs to be justified by giving an example.
Concept Introduction:
The properties of the copper-silver alloy are as follows-
1. Melting point −
2. Molecular weight −
3. Density −
4. Insoluble in water
In the case of silver and copper alloy, both of these are not able to melt together and form a new alloy with
1. Sterling silver − In sterling silver, the weight percentage of pure silver is
2. Coin silver - The weight percentage of pure silver available in coin silver is
There is a new alloy also available known as Electrum. In this alloy, the weight percentage of silver is from
Trending nowThis is a popular solution!

Chapter 11 Solutions
Essentials Of Materials Science And Engineering, Si Edition
- 8-3) Bandpass sampling A bandpass signal is confined to the frequency range from 7.5 to 10.5 kHz. Find the allowed ranges of the sampling rate for this signal. Sketch the amplitude spectrum of a hypothetical message, the amplitude spectrum of the sampled signal, and the transfer function of a suitable recovery filter if the sampling rate is chosen in the center of the lowest range available.arrow_forward8-4) Similar to Lathi & Ding, Prob. P.5.1-5 6.1-4 A low-pass signal g(t) sampled at rate of fs > 2B needs reconstruction. The sampling interval is Ts = 1/fs. (a) If the reconstruction pulse used is p(1) = [1 - specify the equalizer filter E(f) to recover g (1). (b) If the reconstruction pulse used is p(t) = П Ts/2 specify the equalizer filter E(f) to recover g (1).arrow_forward8-2) Lathi & Ding, Prob. P.5.1-1 Determine the Nyquist sampling rate for the following signals, explaining your method: (a) 4 sinc(420лt); (b) 5sinc² (6500лt); (c) sinc(1800лt)+ sinc² (2000лt); (d) 2 sinc(500лt) sin(300л)arrow_forward
- 2) A load consisting of a 1350 Q2 resistor in parallel with a 405 mH inductor is connected across the terminals of a sinusoidal voltage source Vg, where Vg = 90 cos(2500t) V. Find a) the average power delivered to the load, b) the reactive power for the load, c) the apparent power for the load, and d) the power factor of the load.arrow_forward4) Find the phasor voltage Vs for the following circuit if loads L1 and L2 are absorbing 15 kVA at 0.6 pf lagging and 6 kVA at 0.8 pf leading, respectively. Express Vs in polar form. + j10 + 200/0° V(rms) | L1 Li L2arrow_forward3) A 100-V rms, 60 Hz source is applied to a load impedance Z. The apparent power entering the load is 120 VA at a power factor of 0.707 lagging. a) Calculate the complex power b) Find the rms current supplied to the load c) Determine Z d) Assuming that Z = R + jwL, find the values of R and L.arrow_forward
- 1.1 m 1.3 m B 60-mm diameter Brass 40-mm diameter Aluminum PROBLEM 2.52 - A rod consisting of two cylindrical portions AB and BC is restrained at both ends. Portion AB is made of brass (E₁ = 105 GPa, α = 20.9×10°/°C) and portion BC is made of aluminum (Ę₁ =72 GPa, α = 23.9×10/°C). Knowing that the rod is initially unstressed, determine (a) the normal stresses induced in portions AB and BC by a temperature rise of 42°C, (b) the corresponding deflection of point B.arrow_forwardQ1. Choose the correct answer 1. With fixed number of quantization levels in PCM, the quantization noise is (linearly proportional to signal amplitude, non-linearly proportional to signal amplitude, linearly proportional to signal frequency, non-linearly proportional to signal frequency). 2. A PCM encoder uses 130 quantization levels. Which of the following N bits is more economical to encode such signal? (N=6, N=7, N=9, N=10). 3. Frequency Shift Keying can be accomplished by _multiplying two On-Off Keying signals, combining two Frequency Shift Keying signals, adding two (adding two On-Off Keying signals, Frequency Shift Keying signals). 4. Which of the following statements is true with respect to PCM? (The parallel binary data is converted into serial before transmission, Analog data is transmitted directly, Analog signal is amplified before transmission, The analog signal is converted into parallel binary data before transmission). 5. A baseband speech signal of maximum frequency of…arrow_forward30 mm D = 40 MPa -30 mm B C 80 MPa PROBLEM 2.69 A 30-mm square was scribed on the side of a large steel pressure vessel. After pressurization, the biaxial stress condition at the square is as shown. For E = 200 GPa and v=0.30, determine the change in length of (a) side AB, (b) side BC, (c) diagnonal AC.arrow_forward
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





