
(a)
Interpretation:
In the given phase diagram, whether the alloy Al-4% Si is hypo-eutectic or hyper-eutectic needs to be determined.
Concept Introduction:
An alloy is a mixture of metals or the mixture of non-metals with another metal. A metallic bonding character defines alloys. For practical applications, the alloy constituents are generally evaluated by mass proportion and for fundamental science studies in the atomic fraction. Alloys are generally categorized as a replacement or interstitial alloys, based on the alloy's nuclear structure.
Answer to Problem 11.30P
The alloy is hypo-eutectic because its composition lies between eutectic reaction and eutectic composition.
Explanation of Solution
The phase diagram given is as follows:
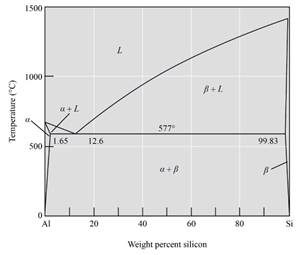
From the phase diagram oftheAl-Si alloy system,
The alloy is hypo-eutectic because the composition is between eutectic reaction and the eutectic composition, and it lies between 1.65% and 12.6%Si.
Thus, from the phase diagram of the Al-Si alloy system, this alloy is hypo-eutectic because the composition lies between eutectic reaction and eutectic composition.
(b)
Interpretation:
The composition of the first solid to form during solidification needs to be determined.
Concept Introduction:
An alloy is a mixture of metals or the mixture of non-metals with another metal. A metallic bonding character defines alloys. For practical applications, the alloy constituents are generally evaluated by mass proportion and for fundamental science studies in the atomic fraction. Alloys are generally categorized as a replacement or interstitial alloys, based on the alloy's nuclear structure.
Answer to Problem 11.30P
The composition of the first form during solidification is 1%.
Explanation of Solution
Given:
It is given that % of Al=96%,wt. of Si=4gm, molar mass of Si=63.54 g/mol, molar mass of Al=26.891g/mol.
Calculations:
The formula for finding the composition of the first solid to form during solidification is,
Putting the values,
Thus,
%Si
Therefore, from the formula, the composition of first solid to form during solidification is 1%.
(c)
Interpretation:
The amounts and compositions of each phase at
Concept Introduction:
An alloy is a mixture of metals or the mixture of non-metals with another metal. A metallic bonding character defines alloys. For practical applications, the alloy constituents are generally evaluated by mass proportion and for fundamental science studies in the atomic fraction. Alloys are generally categorized as a replacement or interstitial alloys, based on the alloy's nuclear structure.
Answer to Problem 11.30P
The amounts and compositions of each phase at
Explanation of Solution
Given Information:
The given phase diagram is as follows:
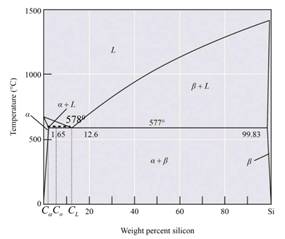
The above-given diagram is the phase diagram of the Al-Si alloy system for the eutectic temperature of
Calculations:
The eutectic reaction of the Al-Si alloy is in the form of
Thus, the liquid phase composition is
Thus, from the given phase diagram of Al-Si the amount and composition is
(d)
Interpretation:
The amounts and compositions at each phase and each micro-constituent at
Concept Introduction:
An alloy is a mixture of metals or the mixture of non-metals with another metal. A metallic bonding character defines alloys. For practical applications, the alloy constituents are generally evaluated by mass proportion and for fundamental science studies in the atomic fraction. Alloys are generally categorized as a replacement or interstitial alloys, based on the alloy's nuclear structure.
Answer to Problem 11.30P
The amounts and compositions of each phase at
Explanation of Solution
GivenInfromation:
The below diagram is the phase diagram of the Al-Si alloy system of eutectic temperature at
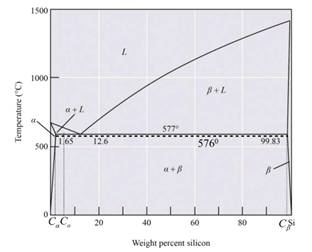
Calculation:
For the Al-Si alloy, the eutectic phase reaction is in the form of
Therefore, the solid phase is calculated as,
The Al-Si alloy contains two stages. Therefore, the Al-Si alloy micro-constituent is primary and eutectic. Solid forming before an alloy is cooled to eutectic temperature is known as the primary micro-constituent and the strong forming is known as eutectic after the cooling temperature. The structure of micro-constituents is the primary
Thus from the phase diagram of Al-Si, the amounts and compositions of each phase at
(e)
Interpretation:
The amounts and compositions at each phase
Concept Introduction:
An alloy is a mixture of metals or the mixture of non-metals with another metal. A metallic bonding character defines alloys. For practical applications, the alloy constituents are generally evaluated by mass proportion and for fundamental science studies in the atomic fraction. Alloys are generally categorized as a replacement or interstitial alloys, based on the alloy's nuclear structure.
Answer to Problem 11.30P
The amounts and compositions of each phase at
Explanation of Solution
Given Information:
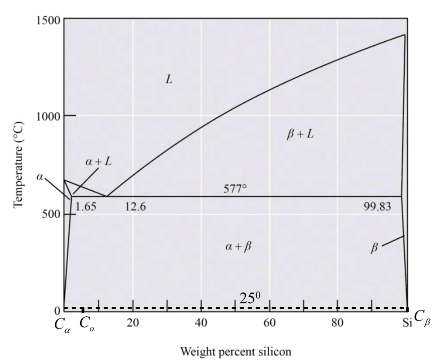
The above diagram is the phase diagram of the Al-Si alloy system of eutectic temperature at
Calculation:
For the Al-Si alloy, the eutectic phase reaction is in the form of
Therefore, the solid phase is calculated as,
Thus from the phase diagram of Al-Si the amounts and compositions of each phase at
Want to see more full solutions like this?
Chapter 11 Solutions
Essentials Of Materials Science And Engineering, Si Edition
- Programming Problems 9.28 Assume that a system has a 32-bit virtual address with a 4-KB page size. Write a C program that is passed a virtual address (in decimal) on the command line and have it output the page number and offset for the given address. As an example, your program would run as follows: ./addresses 19986 Your program would output: The address 19986 contains: page number = 4 offset = 3602 Writing this program will require using the appropriate data type to store 32 bits. We encourage you to use unsigned data types as well. Programming Projects Contiguous Memory Allocation In Section 9.2, we presented different algorithms for contiguous memory allo- cation. This project will involve managing a contiguous region of memory of size MAX where addresses may range from 0 ... MAX - 1. Your program must respond to four different requests: 1. Request for a contiguous block of memory 2. Release of a contiguous block of memory 3. Compact unused holes of memory into one single block 4.…arrow_forwardHomework#5arrow_forwardPlease provide explainations and detailed working. thank youarrow_forward
- using r languagearrow_forwardWrite a function to compute a Monte Carlo estimate of the Beta(3, 3) cdf, and use the function to estimate F(x) for x = 0.1,0.2,...,0.9. Compare the estimates with the values returned by the pbeta function in R.arrow_forwardWrite a function to compute a Monte Carlo estimate of the Gamma(r = 3, λ = 2) cdf, and use the function to estimate F(x) for x = 0.2, 0.4, . . . , 2.0. Compare the estimates with the values returned by the pgamma function in R.arrow_forward
- Oxygen (molar mass 32 kg/kmol) expands reversibly in a cylinder behind a piston at a constant pressure of 3 bar. The volume initially is 0.01 m3 and finally is 0.03 m3; the initial temperature is 17°C. Calculate the work input and the heat supplied during the expansion. Assume oxygen to be an ideal gas and take cp = 0.917 kJ/kg K. For 1 bonus mark explain why (using your understanding of thermodynamics) that oxygen is used in this context rather than water vapour.arrow_forwardThe excitation of a three-phase synchronous motor connected in parallel with a load of 500 kW operating at 0-85 p.f. lagging is adjusted to improve the overall p.f. of the system to 0.95 lagging. If the mechanical load on the motor is 120 kW, calculate the kVA input to the synchronous motor and its p.f.?arrow_forwardusing r languagearrow_forward
- using r languagearrow_forwardHydrodynamic Lubrication Theory Q1: Convert this equations into Python by 1- ah ap a h³ ap 1..ah = ax 12μ ax ay 12μ ay 2 ax Where P=P(x, y) is the oil film pressure. 2- 3μU (L² ε sin P= C²R (1+ cos 0)³ Q2: prove that |h(0) = C(1+ cos 0) ?arrow_forwardA domestic load of 2300 kW at 0.88 p.f lagging and a motors load of 3400 kW at 0.85 p.f lagging are supplied by two alternators operating in parallel. If one alternator is delivering a load of 3300 kW at 0.9 p.f lagging, what will be the output power and p.f of the other alternator?arrow_forward
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





