
(a)
Interpretation:
The product of the reaction of
Concept introduction:
Answer to Problem 11.46AP
The product of the reaction of
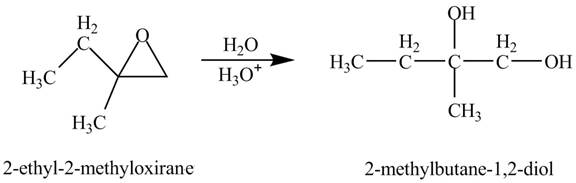
Explanation of Solution
The compound
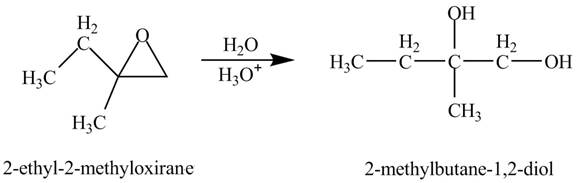
Figure 1
The product of the reaction of
(b)
Interpretation:
The product of the reaction of
Concept introduction:
Epoxides undergo nucleophilic ring opening reactions which are base-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are basic, then the reaction will occur at the less substituted carbon atom.
Answer to Problem 11.46AP
The product of the reaction of
Explanation of Solution
The compound

Figure 2
The product of the reaction of
(c)
Interpretation:
The product of the reaction of
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are base-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are basic, then the reaction will occur at the less substituted carbon atom.
Answer to Problem 11.46AP
The product of the reaction of
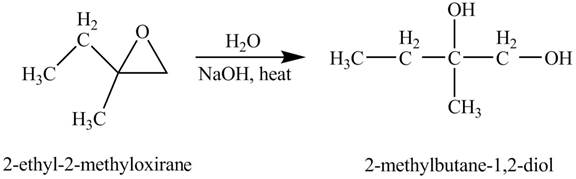
Explanation of Solution
The compound
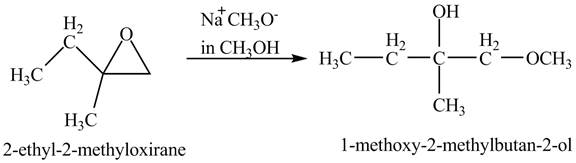
Figure 3
The product of the reaction of
(d)
Interpretation:
The product of the reaction of
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.46AP
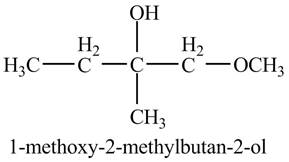
The product of the reaction of
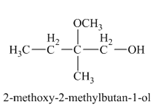
Explanation of Solution
The compound
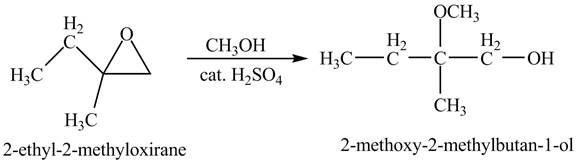
Figure 4
The product of the reaction of
(e)
Interpretation:
The product of the reaction of
Concept introduction:
Epoxides undergo nucleophilic ring opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.46AP
The product of the reaction of
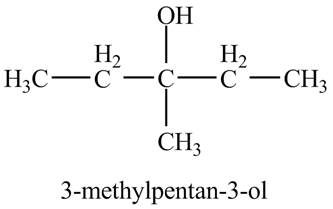
Explanation of Solution
The compound
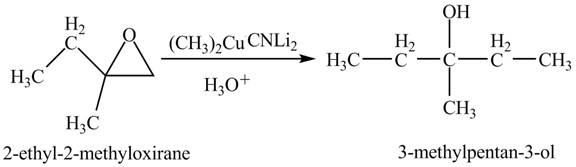
Figure 5
The product of the reaction of
(f)
Interpretation:
The product of the reaction of the product of part (c) with
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.46AP
The product of the reaction of the product of part (c) with
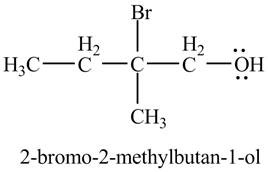
Explanation of Solution
In the presence of
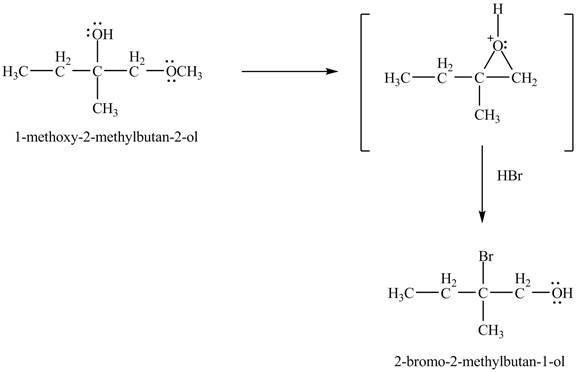
Figure 6
The product of the reaction of the given compound with
(g)
Interpretation:
The product of the reaction of the product of part (d) with
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.46AP
The product of the reaction of the product of part (d) with
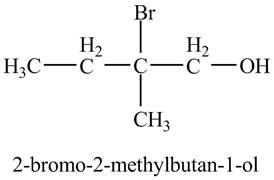
Explanation of Solution
In the presence of
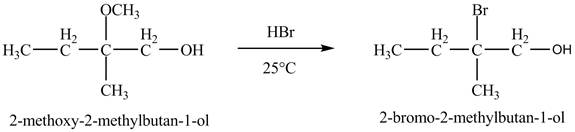
Figure 7
The product of the reaction of the given compound with
(h)
Interpretation:
The product of the reaction of the product of part (c) with
Concept introduction:
The metal hydride reagents are good reducing agents such as
Answer to Problem 11.46AP
The product of the reaction of the product of part (c) with
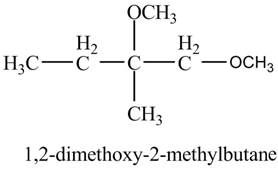
Explanation of Solution
The base
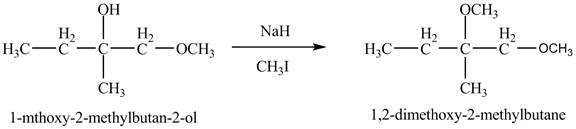
Figure 8
The product of the reaction of the given compound with
(i)
Interpretation:
The product of the reaction of the product of part (d) with
Concept introduction:
The metal hydride reagents are good reducing agents such as
Answer to Problem 11.46AP
The product of the reaction of the product of part (d) with
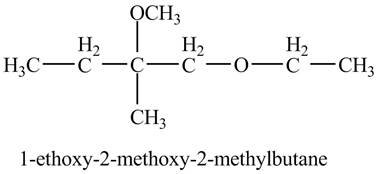
Explanation of Solution
The base
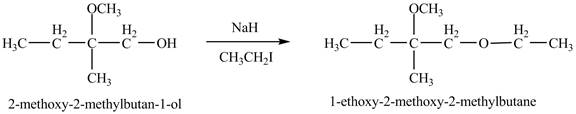
Figure 9
The product of the reaction of the given compound with
(j)
Interpretation:
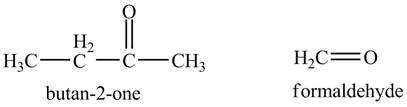
The product of the reaction of the product of part (a) with periodic acid is to be predicted.
Concept introduction:
The periodic acid acts as a strong oxidizing agent. The periodic acid reacts with a vicinal diol to form two
Answer to Problem 11.46AP
The products of the reaction of the product of part (a) with periodic acid are shown below.
Explanation of Solution
The given compound is vicinal diol. It reacts with periodic acid to form two aldehydes. The carbon-carbon bond between the carbon atoms attached to two adjacent hydroxyl groups gets breaks. The corresponding chemical reaction is shown below.
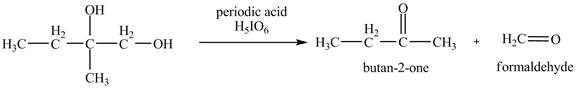
Figure 10
The products of the reaction of the given compound with periodic acid are shown in Figure 10.
(k)
Interpretation:
The product of the reaction of the product of part (f) with
Concept introduction:
Grignard reagents are
Answer to Problem 11.46AP
The product of the reaction of the product of part (f) with
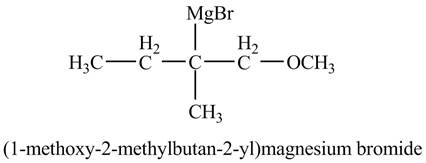
Explanation of Solution
The compound
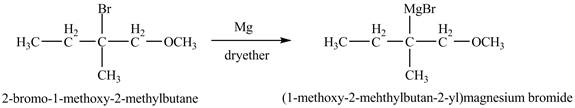
Figure 11
The product of the reaction of the given compound with
(l)
Interpretation:
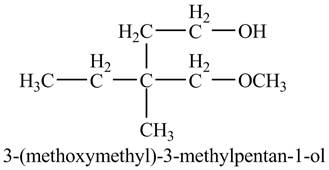
The product of the reaction of the product of part (k) with ethylene oxide followed by addition of
Concept introduction:
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases. Grignard reagents react with carbonyl compounds to form alcohol.
Answer to Problem 11.46AP
The product of the reaction of the product of part (k) with ethylene oxide followed by addition of
Explanation of Solution
Grignard reagent can act as a nucleophile. In the presence of an acid, it can attack the more substituted carbon atom of the epoxy ring. The Grignard reagent reacts with the ethylene oxide followed by protonolysis to form alcohol. The corresponding chemical reaction is shown below.
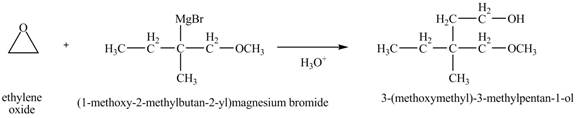
Figure 12
The product of the reaction of the given compound with ethylene oxide followed by addition of
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
- Fill-in-the molecules for the oxidation or reduction of the starting alcohol.arrow_forwardName the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forward
