
Concept explainers
(a)
Interpretation:
The product of the reaction of dibutyl sulfide with
Concept introduction:
Hydrogen perioxide
Sulfur containing organic compounds has many similar chemical properties as oxygen contain compound such as ether and aclcohol.
Answer to Problem 11.45AP
The product of the reaction of dibutyl sulfide with

Explanation of Solution
The dibutyl sulfide undergoes an oxidation reaction with hydrogen peroxide. The dibutyl sulfide reacts with
The corresponding

Figure 1
The product of the reaction of dibutyl sulfide with
(b)
Interpretation:
The product of the reaction of dibutyl sulfide with
Concept introduction:
Hydrogen perioxide
Sulfur containing organic compounds has many similar chemical properties as oxygen contain compound such as ether and aclcohol.
Answer to Problem 11.45AP
The product of the reaction of dibutyl sulfide with
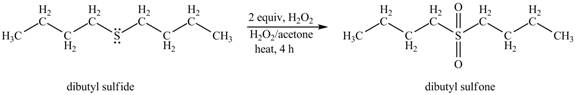
Explanation of Solution
The dibutyl sulfide undergoes an oxidation reaction with hydrogen peroxide. The dibutyl sulfide reacts with
The corresponding chemical reaction is shown below.
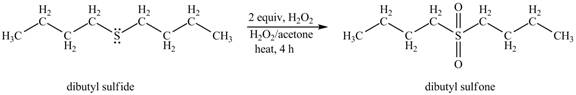
Figure 2
The product of the reaction of dibutyl sulfide with
(c)
Interpretation:
The product of the reaction of
Concept introduction:
The magnesium monoperoxyphthalate
Answer to Problem 11.45AP
The product of the reaction of
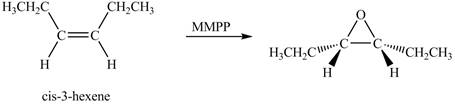
Explanation of Solution
The compound
The corresponding chemical reaction is shown below.
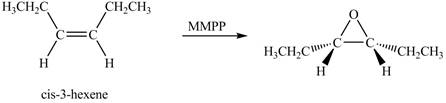
Figure 3
The product of the reaction of
(d)
Interpretation:
The product of the reaction of given compound with
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.45AP
The product of the reaction of given compound with
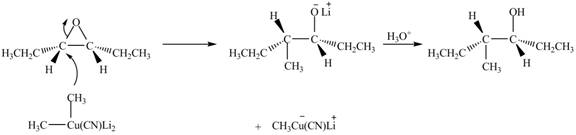
Explanation of Solution
The epoxide undergoes ring-opening reaction in the presence of acid. The dilithium dimethylcyanocuprate molecule generates a nucleophile
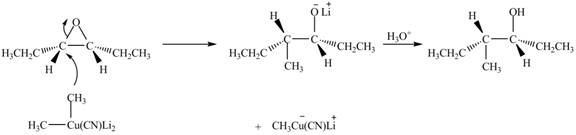
Figure 4
The product of the reaction of given compound with
(e)
Interpretation:
The product of the reaction of given compound with solvent
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.45AP
The product of the reaction of given compound with solvent
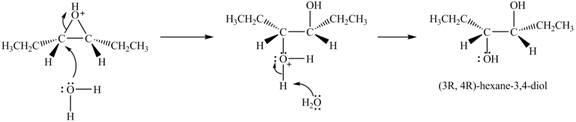
Explanation of Solution
The epoxide undergoes ring opening reaction in the presence of acid. The water molecule acts a nucleophile and attacks on the more substituted carbon atom of the epoxide ring to from

Figure 5
The product of the reaction of given compound with solvent
(f)
Interpretation:
The product of the reaction of given compound with periodic acid is to be predicated.
Concept introduction:
The periodic acid acts as a strong oxidizing agent. The periodic acid reacts with a vicinal diol to form two
Answer to Problem 11.45AP
The product of the reaction of given compound with periodic acid is shown below.
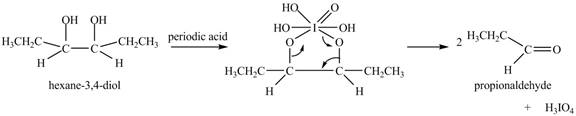
Explanation of Solution
The given compound is vicinal diol. It reacts with periodic acid to form two aldehydes. The carbon-carbon bond between the carbon atoms attached to two adjacent hydroxyl groups gets breaks. The corresponding chemical reaction is shown below.

Figure 6
The product of the reaction of given compound with periodic acid is shown in Figure 6.
(g)
Interpretation:
The product of the reaction of given compound with
Concept introduction:
The metal hydride reagents are good reducing agents such as
Answer to Problem 11.45AP
The product of the reaction of given compound with
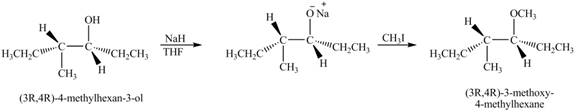
Explanation of Solution
The base
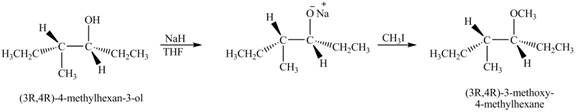
Figure 7
The product of the reaction of given compound with
(h)
Interpretation:
The product of the reaction of
Concept introduction:
Oxymercuration reaction is a type of reaction in which an alkene get converted into an alcohol. The mercuric acetate is used in the reaction as reagent. This reagent attacks the alkene to form a cyclic intermediate compound which further undergoes reduction to form alcohol.
Answer to Problem 11.45AP
The product of the reaction of
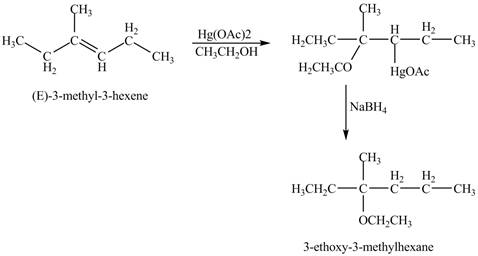
Explanation of Solution
The mercuric acetate
The corresponding chemical reaction is shown below.
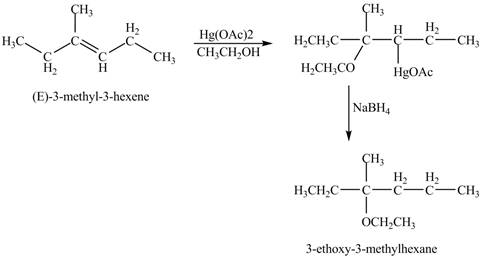
Figure 8
The product of the reaction of
(i)
Interpretation:
The product of the reaction of the given compound with acidic methanol is to be predicated.
Concept introduction:
The replacement or substitution of one
Answer to Problem 11.45AP
The product of the reaction of the given compound with acidic methanol is shown below.
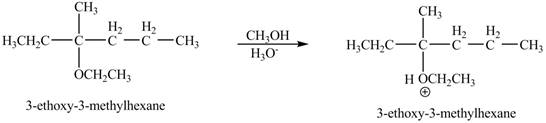
Explanation of Solution
The proton of the acid will protionate the ether. The protonated ether will increase the electrophilic characters of carbon atom attached to the oxygen atom. The methonal can acts as nuclephile, however the nucleophilic charater of methoxy-group and ethoxy group is similar. Therefore, no further reaction will take place.
The corresponding chemical reaction is shown below.
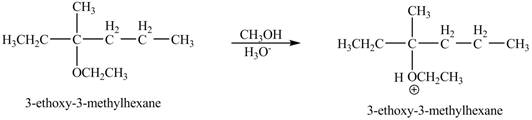
Figure 9
The product of the reaction of the given compound with acidic methanol is shown in Figure 9.
(j)
Interpretation:
The product of the reaction of
Concept introduction:
Grignard reagents are
Answer to Problem 11.45AP
The product of the reaction of
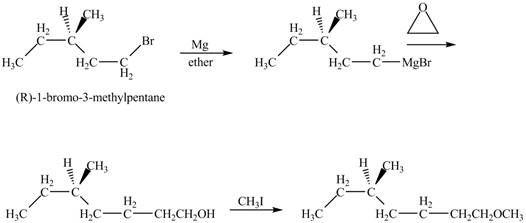
Explanation of Solution
The alkyl halide will react with magnisum metal to from gridnard reagent. These Grignard reagent reacts with epoixde to form alcohol. The alcohol reacts with
The corresponding chemical reaction is shown below.
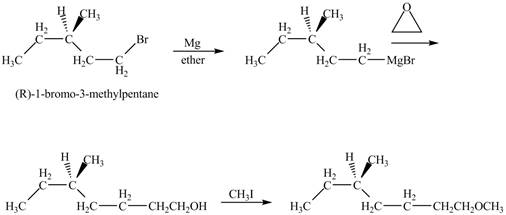
Figure 10
The product of the reaction of
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
- Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forward
- You may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forward
- Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forwardDraw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forward
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





