
(a)
Interpretation:
Given compound has to be named using
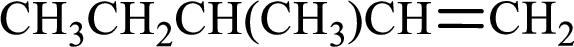
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming
There are about five rules that has to be followed for naming an alkene and an
- The longest continuous carbon chain in the compound that contains double bond or triple has to be identified. This is known as parent compound.
- Suffix “–ane” (in name of
alkane ) is replaced with “-ene” for alkene or “-yne” for alkyne. - Numbering has to be done so that the lowest number is given to the double or triple bond.
- Naming and numbering has to be given for each atom or group that is attached to the parent chain. Numbering has to be done in a way that substituents get the least numbering.
- If the alkenes have more than one double bond they are called as alkadienes (two double bonds) or alkatrienes (three double bonds). Appropriate suffix has to be used depending on the number of multiple bonds present in the compound.
(a)
Explanation of Solution
Given compound is,
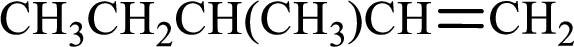
Longest carbon chain with double bond is found to contain five carbon atoms. Therefore, the parent alkane is pentane. As a double bond is present, the alkene name is pentene.
Numbering has to be given in a way that the double bond gets the least numbering. In this case, double bond is present between carbon-1 and carbon-2. Therefore, the parent alkene is 1-pentene.
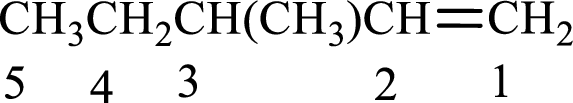
The substituent present in the given structure is a methyl group on carbon-3. Substituents name has to be arranged in the alphabetical order. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkene as 3-methyl-1-pentene.
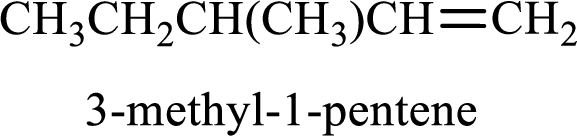
Longest carbon chain containing double bond is pentane. Position of double bond is 1-pentene. Substituents present in the chain are 3-methyl. IUPAC name of the alkene given is 3-methyl-1-pentene.
(b)
Interpretation:
Given compound has to be named using IUPAC nomenclature.
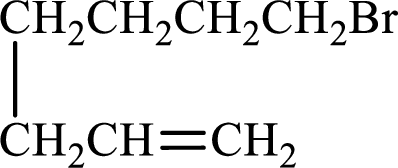
Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
Given compound is,
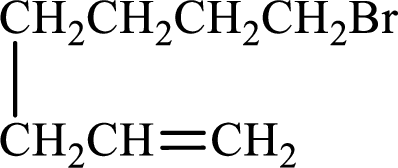
Longest carbon chain with double bond is found to contain seven carbon atoms. Therefore, the parent alkane is heptane. As a double bond is present, the alkene name is heptene.
Numbering has to be given in a way that the double bond gets the least numbering. In this case, double bond is present between carbon-1 and carbon-2. Therefore, the parent alkene is 1-heptene.
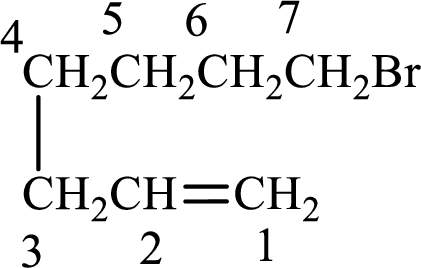
The substituent present in the given structure is a bromine atom on carbon-7. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkene as 7-methyl-1-heptene.
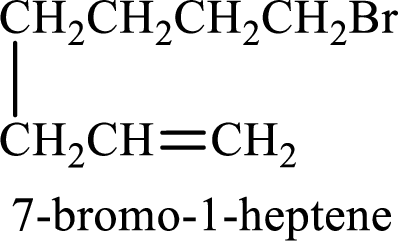
Longest carbon chain containing double bond is heptane. Position of double bond is 1-heptene. Substituents present in the chain are 7-bromo. IUPAC name of the alkene given is 7-bromo-1-heptene.
(c)
Interpretation:
Given compound has to be named using IUPAC nomenclature.
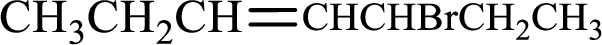
Concept Introduction:
Refer part (a).
(c)
Explanation of Solution
Given compound is,
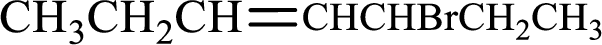
Longest carbon chain with double bond is found to contain seven carbon atoms. Therefore, the parent alkane is heptane. As a double bond is present, the alkene name is heptene.
Numbering has to be given in a way that the double bond gets the least numbering. In this case, double bond is present between carbon-3 and carbon-4. Therefore, the parent alkene is 3-heptene.
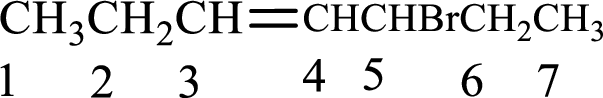
The substituent present in the given structure is a bromine atom on carbon-5. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkene as 5-bromo-3-heptene.
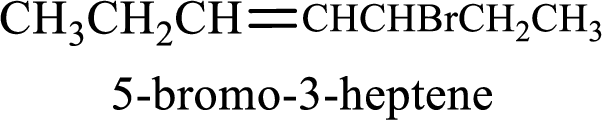
Longest carbon chain containing double bond is heptane. Position of double bond is 3-heptene. Substituents present in the chain are 5-bromo. IUPAC name of the alkene given is 5-bromo-3-heptene.
(d)
Interpretation:
Given compound has to be named using IUPAC nomenclature.
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming cycloalkenes:
- The number of carbon atoms present in the ring is counted and the name of the alkane that has the same number of carbon atoms is given by adding prefix “cyclo-” to the alkane name. Suffix “-ane” is changed as “-ene”.
- The double bond that is present in the ring is given always the number 1.
- If the ring is substituted, then the names of the group or atoms have to be placed before the name of cycloalkene.
- If the ring contains more than one substituent, then the numbers has to be used in a way that it gives the lowest position for the substituents following position 1 for the double bond.
(d)
Explanation of Solution
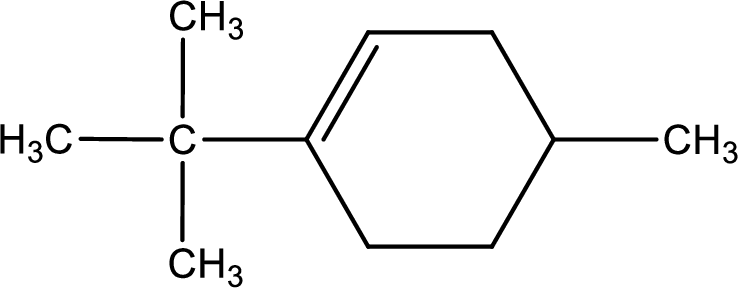
Given compound is,
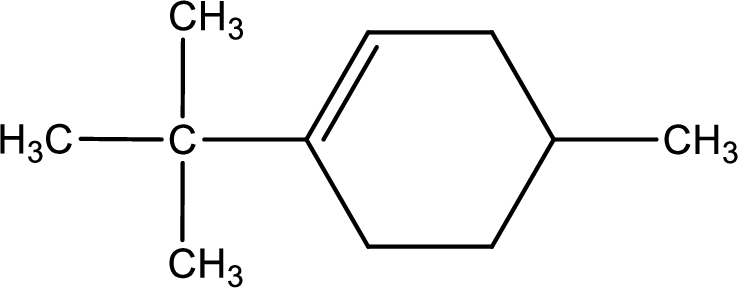
Given compound is found to contain six carbon atoms in a cyclic ring structure. Therefore, the parent cycloalkane is cyclohexane. As there is a double bond present in the ring, the parent compound name is cyclohexene.
Numbering has to be given in a way that the double bond gets the least numbering. In this case, double bond is present between carbon-1 and carbon-2.
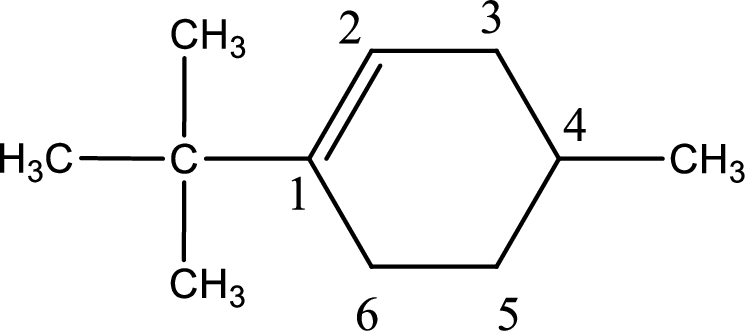
The substituents present in the given structure are a tert-butyl group on carbon-1 and a methyl group on carbon-4. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of compound as 1-tert-butyl-4-methylcyclohexene.
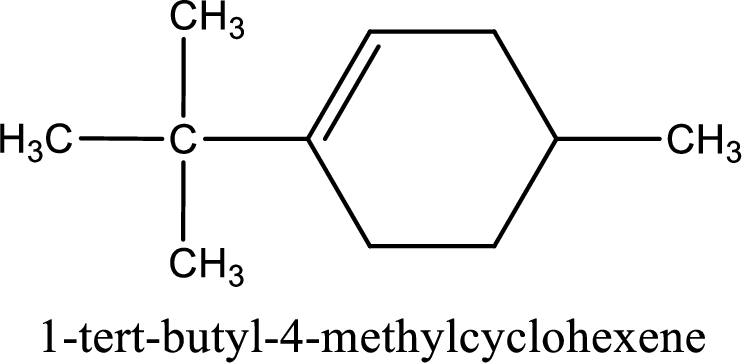
Parent chain is found to be cyclohexene. Position of double bond is carbon-1. Substituent present in the chain is 1-tert-butyl-4-methyl. IUPAC name of the compound given is 1-tert-butyl-4-methylcyclohexene.
Want to see more full solutions like this?
Chapter 11 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY(LL)-PKG
- A common buffer for stabilizing antibodies is 100 mM Histidine at pH 7.0. Describe the preparation of this buffer beginning with L-Histidine monohydrochloride monohydrate and 1 M NaOH. Be certain to show the buffering reaction that includes the conjugate acid and base.arrow_forwardFina x | Sign X Sign X lab: X Intro X Cop) X a chat x My x Grad xLaur x Laur x a sheg X S Shoj XS SHE X acmillanlearning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db27d6b4ee?actualCourseld=d591b3f2- 5 © Macmillan Learning Organic Chemistry Maxwell presented by Macmillan Learning For the dehydrohalogenation (E2) reaction shown, draw the Zaitsev product, showing the stereochemistry clearly. H H KOH Br EtOH Heat Select Draw Templates More Erase // C H Q Search hp Q2 Q Δ קו Resouarrow_forwardIs the structural form shown possible given the pKa constraints of the side chains?arrow_forward
- on x Fina X Sign X Sign x lab X Intro X Cop X chat X My x Grac x Laur x Laur x ashes x S Shox S SHE x a eve.macmillanlearning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db27d6b4ee?actualCourseld=d591b3f2-c stions estion. ct each urces. +95 Macmillan Learning Draw the product formed by the reaction of potassium t-butoxide with (15,25)-1-bromo-2-methyl-1-phenylbutane (shown). Clearly show the stereochemistry of the product. H BH (CH3)3CO-K+ +100 H3CW (CH3)3COH +85 H3CH2C +95 ossible ↓ Q Search Select Draw Templates More C H 0 bp A Erase 2Q 112 Resouarrow_forwardIdentify the structure of the PTH derivative generated after two rounds of Edman degradation.arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 31 0.5 30 26 29 22 28 100 27 33 26 23 15 4 • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. 妊 n ? Previous Nextarrow_forward
- for this question. Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardPLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS!!! PLEASE I UNDERSTAND THE BASICS BUT THIS IS AN EXCEPTION THAT EVEN THE INTERNET CANT HELP!!!! THIS IS THE THIRD TIME I'VE SENT THOSE QUESTIONS SO PLEASE DONT RESEND THE SAME STUFF, ITS NOT HELPING ME!!! I ALSO ALREADY TRIED TO DRAW THE MECHANISM MYSELF, SO IF ITS RIGHT PLEASE TELL ME OR TELL ME WHAT I HAVE TO CHANGE!!! First image: I have to SHOW (DRAWING) the mechanism (with arows and structures of molecules) NOT WORDS PLEASE! of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mechanism (IMAGE) (with arrows and structures of the molecules) NOT WORDS PLEASE !! for the reaction on the left, where the alcohol A is added fast in one portion HOMEWORK, NOT EXAM!! ALL DETAILS ARE IN THE IMAGES PLEASE LOOK AT THE IMAGES, DONT LOOK AT THE AI GENERATED TEXT!!!arrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forward
- Use the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forwardCan I please get help with this? And can I please the lowest possible significant number?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





