
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
9th Edition
ISBN: 9781305968707
Author: Spencer L. Seager
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.33E
Identify the following alkyl groups:
a. 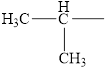
b.
c. 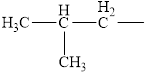
d.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution,
respectively.
F CI
Br |
Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to
have a reasonable yield of product.
NH2
Br
Br
Br
OH
Br
Q7: Rank the following groups in order of basicity, nucleophilicity, and leaving group ability.
a) H₂O, OH, CH3COOT
b) NH3, H₂O, H₂S
Q8: Rank the following compounds in order of increasing reactivity in a nucleophilic substitution
reaction with CN as the nucleophile.
Br
A
B
NH2
LL
F
C
D
OH
CI
LLI
E
Q9: Complete the missing entities for following reactions (e.g., major product(s), reactants,
and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for
reactions a) to d).
a)
H
"Cl
D
+
-OCH 3
Page 3 of 5
Chapter 11 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
Ch. 11 - Prob. 11.1ECh. 11 - Prob. 11.2ECh. 11 - Prob. 11.3ECh. 11 - Prob. 11.4ECh. 11 - Prob. 11.5ECh. 11 - Prob. 11.6ECh. 11 - Prob. 11.7ECh. 11 - Prob. 11.8ECh. 11 - Prob. 11.9ECh. 11 - Prob. 11.10E
Ch. 11 - Prob. 11.11ECh. 11 - Prob. 11.12ECh. 11 - Prob. 11.13ECh. 11 - Prob. 11.14ECh. 11 - What molecular geometry exists when a central...Ch. 11 - Compare the shapes of unhybridized p and...Ch. 11 - Use Example 11.1 and Tables 11.2 and 11.6 to...Ch. 11 - Prob. 11.18ECh. 11 - Prob. 11.19ECh. 11 - Prob. 11.20ECh. 11 - Prob. 11.21ECh. 11 - Prob. 11.22ECh. 11 - Prob. 11.23ECh. 11 - Write a condensed structural formula for the...Ch. 11 - Write a condensed structural formula for the...Ch. 11 - Write an expanded structural formula for the...Ch. 11 - Prob. 11.27ECh. 11 - Classify each of the following compounds as a...Ch. 11 - Why are different conformations of an alkane not...Ch. 11 - Which of the following pairs represent structural...Ch. 11 - Prob. 11.31ECh. 11 - Prob. 11.32ECh. 11 - Identify the following alkyl groups: a. b....Ch. 11 - Prob. 11.34ECh. 11 - Prob. 11.35ECh. 11 - Draw a condensed structural formula for each of...Ch. 11 - Prob. 11.37ECh. 11 - Prob. 11.38ECh. 11 - Prob. 11.39ECh. 11 - Prob. 11.40ECh. 11 - The following names are incorrect, according to...Ch. 11 - The following names are incorrect, according to...Ch. 11 - Prob. 11.43ECh. 11 - Write the correct IUPAC name for each of the...Ch. 11 - Write the correct IUPAC name for each of the...Ch. 11 - Draw the structural formulas corresponding to each...Ch. 11 - Prob. 11.47ECh. 11 - Which of the following pairs of cycloalkanes...Ch. 11 - Prob. 11.49ECh. 11 - Prob. 11.50ECh. 11 - Prob. 11.51ECh. 11 - Which of the following cycloalkanes could show...Ch. 11 - Prob. 11.53ECh. 11 - Using the prefix cis- or trans-, name each of the...Ch. 11 - Prob. 11.55ECh. 11 - The compound decane is a straight-chain alkane....Ch. 11 - Explain why alkanes of low molecular weight have...Ch. 11 - Suppose you have a sample of 2-methylhexane and a...Ch. 11 - Identify circle the alkanelike portions of the...Ch. 11 - Prob. 11.60ECh. 11 - Prob. 11.61ECh. 11 - Write a balanced equation for the incomplete...Ch. 11 - Prob. 11.63ECh. 11 - Prob. 11.64ECh. 11 - Prob. 11.65ECh. 11 - Prob. 11.66ECh. 11 - Prob. 11.67ECh. 11 - Prob. 11.68ECh. 11 - Would you expect a molecule of urea produced in...Ch. 11 - Prob. 11.70ECh. 11 - Prob. 11.71ECh. 11 - Prob. 11.72ECh. 11 - Prob. 11.73ECh. 11 - Prob. 11.74ECh. 11 - Prob. 11.75ECh. 11 - A semi-truck loaded with cyclohexane overturns...Ch. 11 - Prob. 11.77ECh. 11 - Oil spills along coastal shores can be disastrous...Ch. 11 - Prob. 11.79ECh. 11 - Prob. 11.80ECh. 11 - Use the generic formula for alkanes (CnH2n+2) to...Ch. 11 - Prob. 11.82ECh. 11 - Which of the following is an example of an alkane?...Ch. 11 - Prob. 11.84ECh. 11 - Prob. 11.85ECh. 11 - Prob. 11.86ECh. 11 - The deadly property of carbon monoxide, if...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
- Explain why only the lone pairs on the central atom are taken into consideration when predicting molecular shapearrow_forward(ME EX1) Prblm #9/10 Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.arrow_forwardProblems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License