
Concept explainers
Consider a substance X with a ΔHvap = 20.3 kJ/mol and ΔHfus = 9.0 kJ/mol. The melting point, freezing point, and heat capacities of both the solid and liquid X are identical to those of water.
- a If you place one beaker containing 50 g of X at −10°C and another beaker with 50 g of H2O at −10°C on a hot plate and start heating them, which material will reach the boiling point first?
- b Which of the materials from part a, X or H2O, would completely boil away first?
- c On a piece of graph paper, draw the heating curve for H2O and X. How do the heating curves reflect your answers from parts a and b?
(a)
Interpretation:
The substance X with
Explanation of Solution
Explanation
To explain: which material will attain the boiling point earlier when heating
Comparing the given values with water
We should need to compare the vaporization of the given substance and heat of fusion with the values of water. For water,
Heating the substance or water from
In the first step, solid has heated from
Where,
In second step, the solid is melted to liquid at
Where,
In third step, the liquid has heated from
Where,
The heat capacity, temperature change and mass of the substance X is identical to water, the heat required for first and third step are same for both. As the heat of fusion is higher for substance X and in step two, heat required for substance X take longer time. So, water will reach the boiling point earlier than substance X.
(b)
Interpretation:
The substance X with
Explanation of Solution
Explanation
To identify: the material which is totally boiled first from part (a)
Comparing the given values with water
We should need to compare the vaporization of the given substance and heat of fusion with the values of water. For water,
Heating the substance or water from
In the first step, solid has heated from
Where,
In second step, the solid is melted to liquid at
Where,
In third step, the liquid has heated from
Where,
To entirely boil away the substance a further step is needed (step 4).
In fourth step, liquid is boiled to vapour at
As the values of heat of vaporization is much greater than the heat of fusion values. In this step needed much more heat than in step 2 for both water and substance X. Since, heat of vaporisation is less for substance X per mole. Fourth step will require small heat for X and hence it will take less time. The total heat required for fourth step is directly proportional to the time taken for entirely boil away the substance X. However forth step require more time to complete this step. Hence, substance X will boil way first.
(c)
Interpretation:
The substance X with
Explanation of Solution
Explanation
To draw: heating curve for both water and substance X.
Comparing the given values with water
We should need to compare the vaporization of the given substance and heat of fusion with the values of water. For water,
Heating the substance or water from
In the first step, solid has heated from
Where,
In second step, the solid is melted to liquid at
Where,
In third step, the liquid has heated from
Where,
The heating curve for both water and substance X are illustrated below.
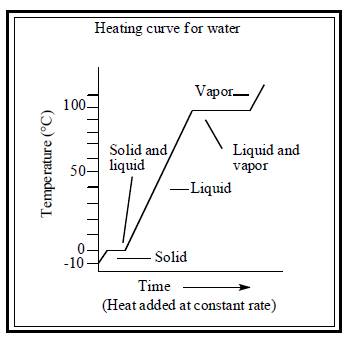
Figure 1
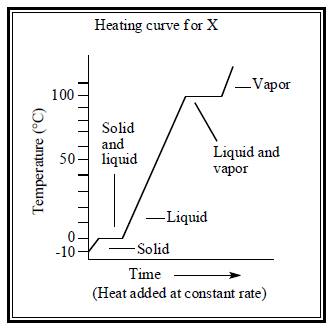
Figure 2
Want to see more full solutions like this?
Chapter 11 Solutions
General Chemistry - Standalone book (MindTap Course List)
- The fire releases 2.80 x 107 Joules of heat energy for each liter of oil burned. The water starts out at 24.5 °C, raising the water's temperature up to 100 °C, and then raises the temperature of the resulting steam up to 325 °C. How many liters of water will be needed to absorb the heat from the fire in this way, for each 1.0 liter of crude oil burned? 4186 J/(kg°C) = heat of water 2020 J/(kg°C) = heat of steam 2,256,000 (i.e. 2.256 x 106) J/kg = latent heat of vaporization for water (at the boiling point of 100 °C).arrow_forward6 Which of the following are likely to be significant resonance structures of a resonance hybrid? Draw another resonance structure for each of the compounds you select as being a resonance form. (A Br: Br: A B C D Earrow_forwardWrite the systematic (IUPAC) name for the following organic molecules. Note for advanced students: you do not need to include any E or Z prefixes in your names. Br structure Br Br Oweuarrow_forward
- Conservation of mass was discussed in the background. Describe how conservation of mass (actual, not theoretical) could be checked in the experiment performed.arrow_forwardWhat impact would adding twice as much Na2CO3 than required for stoichiometric quantities have on the quantity of product produced? Initial results attachedarrow_forwardGiven that a theoretical yield for isolating Calcium Carbonate in this experiment would be 100%. From that information and based on the results you obtained in this experiment, describe your success in the recovery of calcium carbonate and suggest two possible sources of error that would have caused you to not obtain 100% yield. Results are attached form experimentarrow_forward
- 5) Calculate the flux of oxygen between the ocean and the atmosphere(2 pts), given that: (from Box 5.1, pg. 88 of your text): Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturated What is flux if the temperature is 10°C ? (2 pts) (Hint: use the same density in your calculations). Why do your calculated values make sense (or not) based on what you know about the relationship between gas solubility and temperature (1 pt)?arrow_forwardFind a molecular formula for these unknownsarrow_forward(ME EX2) Prblms 8-11 Can you please explain problems 8 -11 to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forward
- Don't used hand raitingarrow_forwardThe following 'H NMR spectrum was taken with a 750 MHz spectrometer: 1.0 0.5 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 ' 2.0 1.0 0.0 (ppm) What is the difference Av in the frequency of RF ac Δν ac radiation absorbed by the a and c protons? (Note: it's not equal to the difference in chemical shifts.) Round your answer to 2 significant digits, and be sure it has an appropriate unit symbol. = O O a will shift left, c will shift right. O a will shift right, c will shift left. a and c will both shift left, with more space between them. Suppose a new spectrum is taken with a 500 MHz spectrometer. What will be true about this new spectrum? O a and c will both shift left, with less space between them. O a and c will both shift right, with more space between them. O a and c will both shift right, with less space between them. Which protons have the largest energy gap between spin up and spin down states? O None of the above. ○ a Ob Explanation Check C Ar B 2025 McGraw Hill LLC. All Rights Reserved.…arrow_forwardWhat mass of Na2CO3 must you add to 125g of water to prepare 0.200 m Na2CO3? Calculate mole fraction of Na2CO3, mass percent, and molarity of the resulting solution. MM (g/mol): Na2CO3 105.99; water 18.02. Final solution density is 1.04 g/mL.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





