
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 11.132QP
Interpretation Introduction
Interpretation:
The liquid water is denser than solid ice. This important to aquatic life and weather has to be explained.
Concept introduction:
- Hydrogen bonding: Hydrogen bonding is weak attractive force that occurs between hydrogen atom bounded to high electronegative atom such as Nitrogen, Oxygen or Fluorine and another adjacent atom having a lone pair of electrons.
- Hydrogen bonding in water molecule: Hydrogen bond involves a hydrogen atom of one water molecule bounded to a lone pair of electrons on Oxygen atom of another molecule of water.
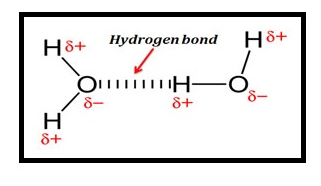
Figure.1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the following reaction with excess reagent, predict the product. Be sure your answer accounts for stereochemistry. If multiple stereocenters are
formed, be sure to draw all products using appropriate wedges and dashes.
1. EtLi, Et₂O
CH₁
?
2. H₂O*
Write the systematic name of each organic molecule:
structure
요
OH
ہو۔
HO
OH
name
X
S
☐
☐
Predict the major products of this organic reaction.
If there aren't any products, because nothing will happen, check the box under the drawing area instead.
D
ㄖˋ
ید
H
No reaction.
+
5
H₂O.*
Click and drag to start
drawing a structure.
OH
H₂O
Chapter 11 Solutions
General Chemistry - Standalone book (MindTap Course List)
Ch. 11.2 - The heat of vaporization of ammonia is 23.4...Ch. 11.2 - Shown here is a representation of a closed...Ch. 11.2 - Prob. 11.2ECh. 11.2 - Selenium tetrafluoride, SeF4, is a colorless...Ch. 11.3 - Prob. 11.4ECh. 11.3 - When camping at high altitude, you need to pay...Ch. 11.5 - Consider two liquids, labeled A and B, that are...Ch. 11.5 - List the different intermolecular forces you would...Ch. 11.5 - Arrange the following hydrocarbons in order of...Ch. 11.5 - At the same temperature, methyl chloride, CH3Cl,...
Ch. 11.5 - A common misconception is that the following...Ch. 11.6 - Prob. 11.8ECh. 11.6 - Prob. 11.9ECh. 11.7 - Figure 11.35 shows solid dots (atoms) forming a...Ch. 11.8 - Shown here is a representation of a unit cell for...Ch. 11.9 - Lithium metal has a body-centered cubic structure...Ch. 11.9 - Potassium metal has a body-centered cubic...Ch. 11 - List the different phase transitions that are...Ch. 11 - Describe how you could purify iodine by...Ch. 11 - Prob. 11.3QPCh. 11 - Explain why 15 g of steam at 100C melts more ice...Ch. 11 - Why is the heat of fusion of a substance smaller...Ch. 11 - Explain why evaporation leads to cooling of the...Ch. 11 - Describe the behavior of a liquid and its vapor in...Ch. 11 - Gases that cannot be liquefied at room temperature...Ch. 11 - Prob. 11.9QPCh. 11 - Why does the vapor pressure of a liquid depend on...Ch. 11 - Prob. 11.11QPCh. 11 - Prob. 11.12QPCh. 11 - Prob. 11.13QPCh. 11 - Prob. 11.14QPCh. 11 - Prob. 11.15QPCh. 11 - Prob. 11.16QPCh. 11 - Prob. 11.17QPCh. 11 - What is the coordination number of Cs in CsCl? of...Ch. 11 - Explain in words how Avogadros number could be...Ch. 11 - Prob. 11.20QPCh. 11 - Prob. 11.21QPCh. 11 - Prob. 11.22QPCh. 11 - Under the right conditions, hydrogen gas, H2, can...Ch. 11 - An element crystallizes with a simple cubic...Ch. 11 - Intermolecular Forces The following picture...Ch. 11 - Heat and Molecular Behavior Part 1: a Is it...Ch. 11 - Shown here is a curve of the distribution of...Ch. 11 - Consider a substance X with a Hvap = 20.3 kJ/mol...Ch. 11 - Prob. 11.29QPCh. 11 - Prob. 11.30QPCh. 11 - Prob. 11.31QPCh. 11 - Prob. 11.32QPCh. 11 - Prob. 11.33QPCh. 11 - Prob. 11.34QPCh. 11 - Prob. 11.35QPCh. 11 - Prob. 11.36QPCh. 11 - Prob. 11.37QPCh. 11 - Prob. 11.38QPCh. 11 - Use Figure 11.7 to estimate the boiling point of...Ch. 11 - Use Figure 11.7 to estimate the boiling point of...Ch. 11 - An electric heater coil provided heat to a 15.5-g...Ch. 11 - Prob. 11.42QPCh. 11 - Isopropyl alcohol, CH3CHOHCH3, is used in rubbing...Ch. 11 - Liquid butane, C4H10, is stored in cylinders to be...Ch. 11 - Water at 0C was placed in a dish inside a vessel...Ch. 11 - A quantity of ice at 0.0C was added to 33.6 g of...Ch. 11 - A quantity of ice at 0C is added to 64.3 g of...Ch. 11 - Steam at 100C was passed into a flask containing...Ch. 11 - Chloroform, CHCl3, a volatile liquid, was once...Ch. 11 - Prob. 11.50QPCh. 11 - White phosphorus, P4, is normally a white, waxy...Ch. 11 - Carbon disulfide, CS2 is a volatile, flammable...Ch. 11 - Prob. 11.53QPCh. 11 - Prob. 11.54QPCh. 11 - Prob. 11.55QPCh. 11 - Prob. 11.56QPCh. 11 - Which of the following substances can be liquefied...Ch. 11 - A tank of gas at 21C has a pressure of 1.0 atm....Ch. 11 - Prob. 11.59QPCh. 11 - Krypton, Kr, has a triple point at 169C and 133...Ch. 11 - Prob. 11.61QPCh. 11 - The heats of vaporization of liquid O2, liquid Ne,...Ch. 11 - For each of the following substances, list the...Ch. 11 - Which of the following compounds would you expect...Ch. 11 - Arrange the following substances in order of...Ch. 11 - Arrange the following substances in order of...Ch. 11 - Methane, CH4, reacts with chlorine, Cl2, to...Ch. 11 - The halogens form a series of compounds with each...Ch. 11 - Prob. 11.69QPCh. 11 - Prob. 11.70QPCh. 11 - List the following substances in order of...Ch. 11 - Arrange the following compounds in order of...Ch. 11 - Classify each of the following by the type of...Ch. 11 - Classify each of the following by the type of...Ch. 11 - Classify each of the following solid elements as...Ch. 11 - Which of the following do you expect to be...Ch. 11 - Prob. 11.77QPCh. 11 - Arrange the following substances in order of...Ch. 11 - Prob. 11.79QPCh. 11 - On the basis of the description given, classify...Ch. 11 - Prob. 11.81QPCh. 11 - Associate each of the solids BN, P4S3, Pb, and...Ch. 11 - Prob. 11.83QPCh. 11 - How many atoms are there in a body-centered cubic...Ch. 11 - Metallic iron has a body-centered cubic lattice...Ch. 11 - Nickel has a face-centered unit cell with all...Ch. 11 - Copper metal has a face-centered cubic structure...Ch. 11 - Barium metal has a body-centered cubic lattice...Ch. 11 - Gold has cubic crystals whose unit cell has an...Ch. 11 - Chromium forms cubic crystals whose unit cell has...Ch. 11 - Assume X has a body-centered cubic lattice with...Ch. 11 - Lead has a face-centered cubic lattice with all...Ch. 11 - Prob. 11.93QPCh. 11 - Metallic barium has a body-centered cubic...Ch. 11 - Prob. 11.95QPCh. 11 - Prob. 11.96QPCh. 11 - Prob. 11.97QPCh. 11 - Prob. 11.98QPCh. 11 - Prob. 11.99QPCh. 11 - Prob. 11.100QPCh. 11 - Prob. 11.101QPCh. 11 - Prob. 11.102QPCh. 11 - Describe the formation of hydrogen bonds in...Ch. 11 - Prob. 11.104QPCh. 11 - Ethylene glycol (CH2OHCH2OH) is a slightly viscous...Ch. 11 - Pentylamine, CH3CH2CH2CH2CH2NH2, is a liquid that...Ch. 11 - Prob. 11.107QPCh. 11 - Prob. 11.108QPCh. 11 - Decide which substance in each of the following...Ch. 11 - Prob. 11.110QPCh. 11 - Iridium metal, Ir, crystallizes in a face-centered...Ch. 11 - The edge length of the unit cell of tantalum...Ch. 11 - Prob. 11.113QPCh. 11 - Rubidium metal has a body-centered cubic structure...Ch. 11 - Calculate the percent of volume that is actually...Ch. 11 - Calculate the percent of volume that is actually...Ch. 11 - For the hydrogen halides and the noble gases, we...Ch. 11 - For the carbon and nitrogen family hydrides, we...Ch. 11 - Prob. 11.119QPCh. 11 - Prob. 11.120QPCh. 11 - Prob. 11.121QPCh. 11 - Prob. 11.122QPCh. 11 - Prob. 11.123QPCh. 11 - Prob. 11.124QPCh. 11 - A geckos toes have been shown to stick to walls...Ch. 11 - Prob. 11.126QPCh. 11 - Prob. 11.127QPCh. 11 - Prob. 11.128QPCh. 11 - Prob. 11.129QPCh. 11 - Prob. 11.130QPCh. 11 - Prob. 11.131QPCh. 11 - Prob. 11.132QPCh. 11 - In an experiment, 20.00 L of dry nitrogen gas, N2,...Ch. 11 - On a particular summer day, the temperature is...Ch. 11 - Prob. 11.135QPCh. 11 - Prob. 11.136QPCh. 11 - Prob. 11.137QPCh. 11 - Prob. 11.138QPCh. 11 - Prob. 11.139QPCh. 11 - Prob. 11.140QPCh. 11 - Rhenium forms a series of solid oxides: Re2O7...Ch. 11 - Shown below is the cubic unit cell of an ionic...Ch. 11 - Prob. 11.143QPCh. 11 - Strontium crystallizes as a face-centered cubic...Ch. 11 - Prob. 11.145QPCh. 11 - Prob. 11.146QPCh. 11 - Prob. 11.147QPCh. 11 - Prob. 11.148QPCh. 11 - Prob. 11.149QPCh. 11 - Prob. 11.150QPCh. 11 - Prob. 11.151QPCh. 11 - Prob. 11.152QPCh. 11 - How much heat must be added to 28.0 g of solid...Ch. 11 - Prob. 11.154QPCh. 11 - Prob. 11.155QPCh. 11 - Prob. 11.156QPCh. 11 - Nanotechnology, or technology utilizing 1100 nm...
Knowledge Booster
Similar questions
- Draw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction 'O 10 + x 也 HO + 义 Click and drag to start drawing a structure.arrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? H- :0: C=N: b Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0° b=0 Xarrow_forwardA student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. • If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + This transformation can't be done in one step. T iarrow_forward
- Determine the structures of the missing organic molecules in the following reaction: H+ O OH H+ + H₂O ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. X § ©arrow_forwardTable 1.1 Stock Standard Solutions Preparation. The amounts shown should be dissolved in 100 mL. Millipore water. Calculate the corresponding anion concentrations based on the actual weights of the reagents. Anion Amount of reagent (g) Anion Concentration (mg/L) 0.1649 Reagent Chloride NaCl Fluoride NaF 0.2210 Bromide NaBr 0.1288 Nitrate NaNO3 0.1371 Nitrite NaNO2 0.1500 Phosphate KH2PO4 0.1433 Sulfate K2SO4 0.1814arrow_forwardDraw the structure of the pound in the provided CO as a 300-1200 37(2), 11 ( 110, and 2.5 (20arrow_forward
- Please help me with # 4 and 5. Thanks in advance!arrow_forwardA small artisanal cheesemaker is testing the acidity of their milk before it coagulates. During fermentation, bacteria produce lactic acid (K₁ = 1.4 x 104), a weak acid that helps to curdle the milk and develop flavor. The cheesemaker has measured that the developing mixture contains lactic acid at an initial concentration of 0.025 M. Your task is to calculate the pH of this mixture and determine whether it meets the required acidity for proper cheese development. To achieve the best flavor, texture and reduce/control microbial growth, the pH range needs to be between pH 4.6 and 5.0. Assumptions: Lactic acid is a monoprotic acid H H :0:0: H-C-C H :0: O-H Figure 1: Lewis Structure for Lactic Acid For simplicity, you can use the generic formula HA to represent the acid You can assume lactic acid dissociation is in water as milk is mostly water. Temperature is 25°C 1. Write the K, expression for the dissociation of lactic acid in the space provided. Do not forget to include state symbols.…arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :0: :0 H. 0:0 :0: :6: S: :0: Select to Edit Arrows ::0 Select to Edit Arrows H :0: H :CI: Rotation Select to Edit Arrows H. < :0: :0: :0: S:arrow_forward
- 3:48 PM Fri Apr 4 K Problem 4 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Mg. :0: Select to Add Arrows :0: :Br: Mg :0: :0: Select to Add Arrows Mg. Br: :0: 0:0- Br -190 H 0:0 Select to Add Arrows Select to Add Arrows neutralizing workup H CH3arrow_forwardIarrow_forwardDraw the Markovnikov product of the hydrobromination of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. + Explanation Check 1 X E 4 1 1 1 1 1 HBr Click and drag to start drawing a structure. 80 LE #3 @ 2 $4 0 I அ2 % 85 F * K M ? BH 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center & 6 27 FG F10 8 9 R T Y U D F G H P J K L Z X C V B N M Q W A S H option command H command optiarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning