
CHEMISTRY-TEXT
8th Edition
ISBN: 9780134856230
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 11.24SP
The chemical structure for oleic acid, the primary component of olive oil, is shown. Explain why olive oil has a higher viscosity than water. 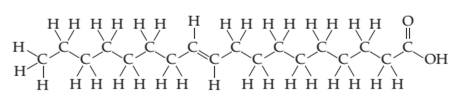
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
QUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression'
*The images of the data showing 'coefficients for the standard curve' have been provided
Using the Nernst equation to calculate nonstandard cell voltage
Try Again
Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations.
A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
2+
2+
Sn²+ Ba(s)
(aq) + Ba (s) Sn (s) + Ba²+ (aq)
→>>
Suppose the cell is prepared with 6.10 M Sn
2+
2+
in one half-cell and 6.62 M Ba
in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.
1.71 V
☐ x10
☑
5
0/5
?
00.
18
Ar
Question: Find both the b (gradient) and a (y-intercept) value from the list of data below:
(x1 -x̄)
370.5
(y1 - ȳ)
5.240
(x2 - x̄)
142.5
(y2 - ȳ)
2.004
(x3 - x̄)
28.5
(y3 - ȳ)
0.390
(x4 - x̄)
-85.5
(y4 - ȳ)
-1.231
(x5 - x̄)
-199.5
(y5 - ȳ)
-2.829
(x6 - x̄)
-256.5
(y6 - ȳ)
-3.575
Chapter 11 Solutions
CHEMISTRY-TEXT
Ch. 11 - Prob. 11.1PCh. 11 - The normal boiling point of water is 100.0 C, and...Ch. 11 - PRACTICE 11.1 The boiling point of ethanol is 78.4...Ch. 11 - APPLY 11.2 Chloroform CHCl3 has Hvap=29.2kJ/mol...Ch. 11 - How much heat is required to convert15.0 g of...Ch. 11 - APPLY 11.4 What is the sign and magnitude of q...Ch. 11 - Look at the phase diagram of H2O in Figure 11.7,...Ch. 11 - Prob. 11.8ACh. 11 - Why was a new solvent needed for extracting...Ch. 11 - A fire extinguisher containing carbon dioxide has...
Ch. 11 - Look at the phase diagram of CO2 in Figure11.13,...Ch. 11 - Liquid carbon dioxide is also used as non-toxic...Ch. 11 - For the phase transition CO2(s)CO2(g), predict the...Ch. 11 - A sample of supercritical carbon dioxide was...Ch. 11 - Assume that you have a liquid in a cylinder...Ch. 11 - The phase diagram of a substance is shown below....Ch. 11 - Prob. 11.17CPCh. 11 - Prob. 11.18CPCh. 11 - The following compound undergoes a phase...Ch. 11 - A magnetized needle gently placed on the surface...Ch. 11 - Water flows quickly through the narrow neck of a...Ch. 11 - Predict which substance in each pair has the...Ch. 11 - Prob. 11.23SPCh. 11 - The chemical structure for oleic acid, the primary...Ch. 11 - Prob. 11.25SPCh. 11 - Prob. 11.26SPCh. 11 - The vapor pressure of SiCI4 is 100 mm Hg at 5.4 C,...Ch. 11 - What is the vapor pressure of CS2 in mm Hg at 20.0...Ch. 11 - What is the vapor pressure of SiCI4 in mm Hg at...Ch. 11 - Dichloromethane, CH2CI2, is an organic solvent...Ch. 11 - Prob. 11.31SPCh. 11 - Use the plot you made in Problem 11.30 to find a...Ch. 11 - Prob. 11.33SPCh. 11 - Prob. 11.34SPCh. 11 - Prob. 11.35SPCh. 11 - Prob. 11.36SPCh. 11 - Acetone,acommon laboratorysolvent,has...Ch. 11 - Why is Hvap usually larger than Hfusion ?Ch. 11 - Why is the heat of sublimation, Hsubl, equal to...Ch. 11 - Naphthalene, better known as "mothballs," has bp =...Ch. 11 - Prob. 11.41SPCh. 11 - Prob. 11.42SPCh. 11 - Prob. 11.43SPCh. 11 - Prob. 11.44SPCh. 11 - Prob. 11.45SPCh. 11 - How much energy in kilojoules is needed to heat...Ch. 11 - Prob. 11.47SPCh. 11 - How much energy in kilojoules is released when...Ch. 11 - How much energy in kilojoules is released when...Ch. 11 - Prob. 11.50SPCh. 11 - Prob. 11.51SPCh. 11 - Prob. 11.52SPCh. 11 - Prob. 11.53SPCh. 11 - Prob. 11.54SPCh. 11 - Look at the phase diagram of H2O in Figure 11.7,...Ch. 11 - Prob. 11.56SPCh. 11 - Oxygen has Tt=54.3K,Pt=1.14mmHg,Tc=154.6K, and...Ch. 11 - Prob. 11.58SPCh. 11 - Prob. 11.59SPCh. 11 - Prob. 11.60SPCh. 11 - Prob. 11.61SPCh. 11 - Benzene has a melting point of 5.53 C and a...Ch. 11 - Prob. 11.63SPCh. 11 - How many phase transitions did you pass through in...Ch. 11 - Prob. 11.65SPCh. 11 - Prob. 11.66SPCh. 11 - Prob. 11.67SPCh. 11 - Prob. 11.68SPCh. 11 - Prob. 11.69SPCh. 11 - Prob. 11.70SPCh. 11 - Prob. 11.71SPCh. 11 - Prob. 11.72SPCh. 11 - Prob. 11.73SPCh. 11 - For each of the following substances, identify the...Ch. 11 - The chlorofluorocarbon refrigerant...Ch. 11 - Prob. 11.76MPCh. 11 - Prob. 11.77MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forwardThe following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forward
- The following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forwardBriefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forward
- Explain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0 262.7 QUESTION: For both groups of data provide answers to the calculations attached in the imagearrow_forward
- 7. Concentration and uncertainty in the estimate of concentration (class data) Class mean for sample (Regular) |[Cl-] (mmol/L) class mean Sn za/2 95% Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Confidence Interval (mg/100 mL)arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardGive reason(s) for six from the followings [using equations if possible] a. Addition of sodium carbonate to sulfanilic acid in the Methyl Orange preparation. b. What happened if the diazotization reaction gets warmed up by mistake. c. Addition of sodium nitrite in acidified solution in MO preparation through the diazotization d. Using sodium dithionite dihydrate in the second step for Luminol preparation. e. In nitroaniline preparation, addition of the acid mixture (nitric acid and sulfuric acid) to the product of step I. f. What is the main reason of the acylation step in nitroaniline preparation g. Heating under reflux. h. Fusion of an organic compound with sodium. HAND WRITTEN PLEASEarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Viscosity, Cohesive and Adhesive Forces, Surface Tension, and Capillary Action; Author: Professor Dave Explains;https://www.youtube.com/watch?v=P_jQ1B9UwpU;License: Standard YouTube License, CC-BY